Chapter: 10th Science : Chapter 8 : Periodic Classification of Elements
Periodic Trends in Properties
PERIODIC TRENDS IN
PROPERTIES
The electronic
configurations of elements help us to explain the periodic recurrence of
physical and chemical properties. Anything which repeats itself after a regular
interval is called periodic and this behaviour is called periodicity.
Some of the atomic properties of the elements are periodic.
Properties such as
atomic radius, ionic radius, ionisation energy, electronegativity, electron
affinity, show a regular periodicity and hence they are called periodic
properties. The main significance of the modern periodic table is that it
gives a clear understanding of the general properties and trends within a group
or a period to predict with considerable accuracy, the properties of any
element, even though that element may be unfamiliar to us. Let us discuss the
periodic trend of some of the properties.
1. Atomic Radius
Atomic radius of an atom is defined as
the distance between the centre of its nucleus and the outermost shell
containing the valence electron. Direct measurement of the radius of an
isolated atom is not possible. Except for noble gases, usually the atomic
radius is referred to as covalent radius or metallic radius depending
on the nature of the bonding between the concerned atoms.Atomic radius in metal
atoms is known as metallic radius.It is defined as half the distance between
the nuclei of adjacent metal atoms (Figure 8.2 (a)).
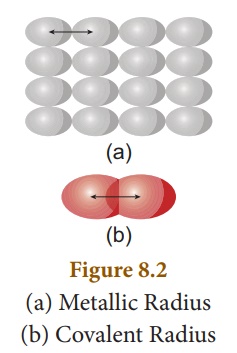
In non-metallic elements, their atomic radius is known as Covalent radius. It is defined as half the distance between the nuclei of two covalently bonded atoms of the same element in a molecule (Figure 8.2 (b)). For example, let us consider H2 molecule. The distance between the two hydrogen nuclei of the molecule is 0.74 Å. So its covalent radius is 0.74/2 = 0.37 Å.
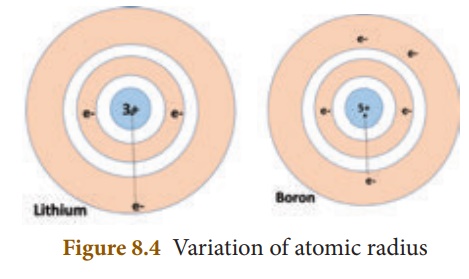
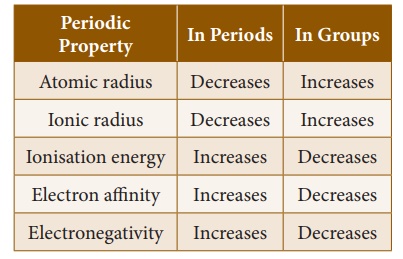
When you look at the variation of the atomic radii in the periodic table, there are two distinct trends. Along the period, from left to right, the
atomic radius of the elements decreases whereas along the groups, from the top
to bottom, the atomic radius increases. The increase, down a group, is due to
the increase in the valence shell number down the group. As the shell number
increases, the distance between the valence shell and the nucleus increases. In
contrast, when you observe along the period, the shell number remains the same
but the number of protons (i.e. atomic number) increases. More and more
positive charges impose a strong attraction over the electrons and thus the
electron cloud shrinks towards the nucleus, which results in the decrease in
the atomic size. Figure 8.4 shows how the atomic radius decreases from lithium
to boron.
2. Ionic Radii
It is defined as the
distance from the centre of the nucleus of the ion upto the point where it
exerts its influence on the electron cloud of
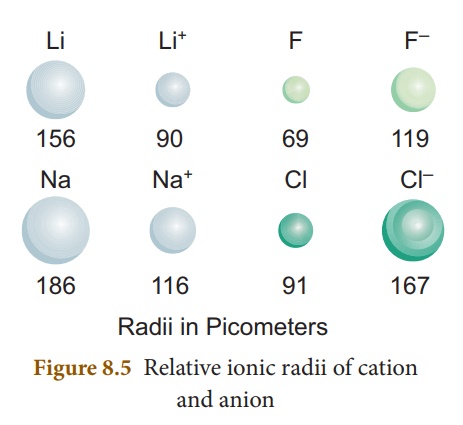
You know that
ions are formed when an atom lose or gain electrons. When a neutral atom loses
an electron, it becomes a positively charged ion called cation, whereas
the gain of an electron by a neutral atom forms a negatively charged ion called
anion. The size of the ions is important to determine their behaviours
in solutions and the structure of ionic solids. The size of a cation is always
smaller than its corresponding neutral atom. But, the anion is larger than its
neutral atom.
For instance, lithium
and sodium lose the single electron from their outermost energy level to form
cations. The ions so formed are smaller because the remaining electrons are at
a inner cells and attracted more strongly by the nucleus. Fluorine and chlorine
become negative ions by gaining an electron. When electrons are added, the charge
on the nucleus is not great enough to hold the increased number of electrons as
closely as it holds the electrons in the neutral atom. So, as seen in atomic
radius, ionic radii also decrease along the period from left to right
and increase down the group.
3. Ionisation Energy
Ionisation energy is the
minimum energy required to remove an electron from a gaseous atom in its ground
state to form a cation. It is otherwise called ionisation enthalpy. It
is measured in kJ/mol. Higher the ionisation energy, it is more difficult to
remove the electron.
As the atomic size
decreases from left to right in a period, more energy is required to remove the
electrons. So, the ionisation energy increases along the period. But,
down the group, the atomic size increases and hence the valence
electrons are loosely bound. They require relatively less energy for the
removal. Thus, ionisation energy decreases down the group in the
periodic table.
Note: As the positive charge
increases the size of the cation decreases
As the negative charge
increases the size of the anion increases
4. Electron Affinity
Electron affinity is the
amount of energy released when a gaseous atom gains an electron to form its
anion. It is also measured in kJ/mol and represented by the following equation:
A(g) + e–
→ A–(g) + Energy
Cl(g) + e–
→ Cl–(g) + energy
Like ionisation energy,
electron affinity also increases from left to right in a period and decreases
from top to bottom in a group.
5. Electronegativity
Electronegativity of an
element is the measure of the tendency of its atom to attract the shared pair
of electrons towards itself in a covalent bond. Let us consider HCl molecule.
Both the hydrogen and chlorine atoms share one electron each to form the
covalent bond between them. chlorine atom has a higher electronegativity and
hence it pulls the shared electrons towards itself more strongly than hydrogen.
Thus, when the bond breaks, the bonding electrons are left with chlorine
forming H+ and Cl– ions. It is represented,
diagrammatically, as shown below:
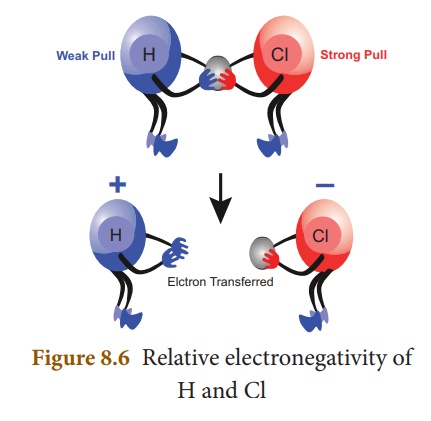
Electronegativity is
based on various experimental data such as bond energy, ionization potential,
electron affinity, etc.
Pauling scale is the
widely used scale to determine the electronegativity, which in turn predicts
the nature of bonding (ionic or covalent) between the atoms in a molecule.
Electronegativity of
some of the elements are given below
F = 4.0, Cl = 3.0, Br =
2.8, I = 2.5, H = 2.1, Na = 1
If the difference in
electronegativity between two elements is 1.7, the bond has 50% ionic character
and 50% covalent character.
If the difference is
less than 1.7, the bond is considered to be covalent.
If the difference is
greater than 1.7, the bond is considered to be ionic.
Along the period, from
left to right in the periodic table, the electronegativity increases because of
the increase in the nuclear charge which in turn attracts the electrons more
strongly. On moving down a group, the electronegativity of the elements
decreases because of the increased number of energy levels.
Related Topics