Chapter: Modern Pharmacology with Clinical Applications: β-Lactam Antibiotics
Penicillins
PENICILLINS
The penicillins are a large
group of bactericidal com-pounds. They can be subdivided and classified by
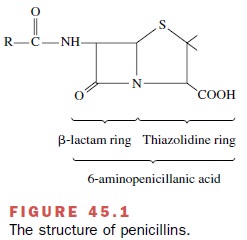
their chemical structure and spectrum of activity. The struc-ture common to all
penicillins is a β-lactam ring fused with a thiazolidine nucleus (Fig. 45.1).
The antimicrobial activity of penicillin resides in the β-lactam ring.
Splitting of the β-lactam ring by either acid hydrolysis or β-lactamases
results in the formation of penicilloic acid, a product without antibiotic
activity. Addition of various side chains (R) to the basic penicillin molecule creates
classes of compounds with the same mechanism of action as penicillin but with
different chemical and biological properties. For example, some analogues are
resistant to hydrolysis by acid or β-lactamase; some have an extended the
spectrum of antibacterial activity; and others show improved absorption from
the intes-tinal tract.
Penicillins may be classified
into four groups: natural penicillins (G and V), antistaphylococcal
(penicillinase-resistant) penicillins, aminopenicillins, and antipseudo-monal
penicillins. Natural penicillins have therapeutic ef-fects limited to
streptococci and a few gram-negative organisms. The antistaphylococcal
(penicillinase-resist-ant) penicillins treat infections caused by streptococci
and staphylococci but do not affect MRSA. The amino-penicillins are effective
against streptococci, enterococci, and some gram-negative organisms but have
variable activity against staphylococci and are ineffective against P. aeruginosa. The antipseudomonal
penicillins retain activity against
streptococci and possess additional ef-fects against gram-negative organisms,
including various Enterobacteriaceae and Pseudomonas.
Natural Penicillins
Penicillin G
(benzylpenicillin) is an acid-labile com-pound having variable bioavailability
after oral adminis-tration. Consequently, penicillin G is most appropriate for
intramuscular or intravenous therapy. The drug dis-tributes to most tissues and
serosa-lined cavities, al-though low concentrations appear in breast milk and cerebrospinal
fluid. When the meninges are inflamed, cerebrospinal fluid concentrations of
penicillin G ap-proximate 5% of the serum concentration. In inflamed joints,
concentrations of the drug approach serum levels.
Penicillin G is excreted by
the kidneys, with 90% of renal elimination occurring via tubular secretion and
10% by glomerular filtration. Probenecid blocks tubular secretion and has been
used to increase the serum con-centration and prolong the half-life of
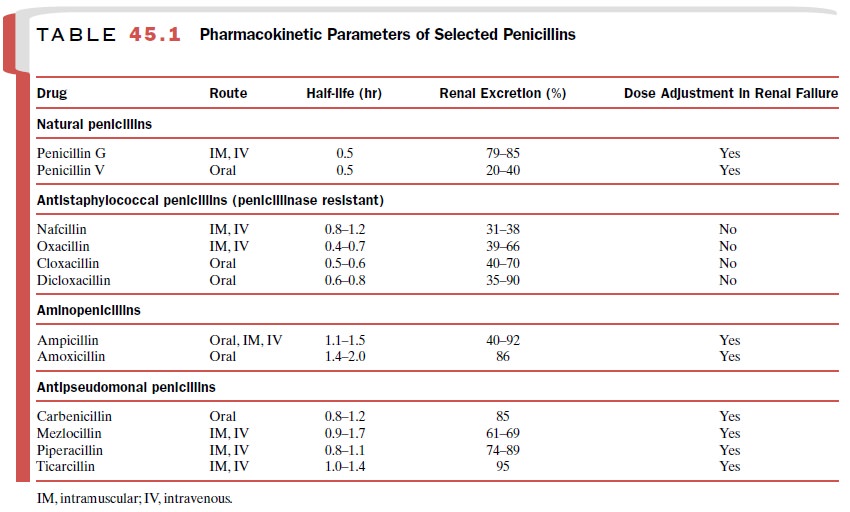
penicillin G and other penicillins. Additional pharmacokinetic informa-tion can
be found in Table 45.1.
The clinical uses of
penicillin G include endocarditis caused by S.
viridans (or Streptococcus bovis),
pharyngi-tis (group A -hemolytic streptococci), cat bite cellulitis (Pasteurella multocida), and syphilis (Treponema pal-lidum).
Depot intramuscular
formulations of penicillin G, including procaine penicillin and benzathine
penicillin, have decreased solubility, delayed absorption, and a prolonged
half-life. Drug concentrations are detectable 24 hours after injection of
procaine penicillin, and low levels of benzathine penicillin (0.003 units/mL)
are de-tectable 4 weeks after injection.
When prescribing one of the
penicillin G depot for-mulations, practitioners must individualize treatment to
clinical and microbial conditions. Some long-acting for-mulations may not
maintain adequate plasma and tissue concentrations to treat specific organisms
or infections. For acute streptococcal meningitis, the goal is rapid achievement
of high antibiotic concentrations in the cerebrospinal fluid. Consequently,
depot formulations are inappropriate for meningitis. Intravenous penicillin G
is among the antibiotics of first choice for therapy of meningitis caused by
susceptible S. pneumoniae. In
con-trast, a depot formulation of benzathine penicillin G suffices for
rheumatic fever prophylaxis.
Penicillin V is an orally
administered phenoxy-methyl congener of penicillin G having an antibacterial
spectrum of activity that is similar to that of penicillin G. Penicillin V is
used to treat streptococcal infections when oral therapy is appropriate and
desirable.
Antistaphylococcal (penicillinase-resistant) Penicillins
Nafcillin, oxacillin, cloxacillin, and dicloxacillin are more resistant to bacterial β-lactamases than is penicillin G. Consequently, these antibiotics are effective against streptococci and most community-acquired penicilli-nase-producing staphylococci. Methicillin, which is no longer marketed in the United States, is another peni-cillinase-resistant antibiotic similar to nafcillin and oxacillin. For historical reasons, staphylococci resistant to oxacillin or nafcillin are labeled methicillin resistant. Many hospitals are reservoirs for MRSA and methi-cillin-resistant Staphylococcus epidermidis (MRSE). These nosocomial pathogens are resistant in vitro to all β-lactam antibiotics.
For parenteral therapy,
nafcillin and oxacillin offer comparable efficacy and antimicrobial spectra of
activ-ity. Although both drugs undergo hepatic metabolism, only nafcillin
requires dose adjustment in patients with combined hepatic and renal
insufficiency. Other phar-macokinetic data for nafcillin and oxacillin appear
in Table 45.1. Indications for nafcillin or oxacillin include severe
staphylococcal infections like cellulitis, empyema, endocarditis,
osteomyelitis, pneumonia, septic arthritis, and toxic shock syndrome.
For oral therapy, cloxacillin
and dicloxacillin are comparable alternatives. Both undergo hepatic
metabo-lism, and neither drug requires dose adjustment in pa-tients with
hepatic insufficiency. Additional pharmaco-kinetic data are in Table 45.1.
Indications for cloxacillin or dicloxacillin include clinically mild
staphylococcal in-fections like impetigo.
Aminopenicillins
The pharmacokinetics of
ampicillin and amoxicillin are similar (Table 45.1). Both have good oral
bioavailabil-ity; ampicillin is also bioavailable after intramuscular
injection. Concomitant ingestion of food decreases the bioavailability of
ampicillin but not amoxicillin. Consequently, oral doses of ampicillin should
be given on an empty stomach. Ampicillin achieves therapeutic concentrations in
the cerebrospinal fluid only during in-flammation. Therefore, ampicillin is
effective treatment for meningitis caused by Listeria monocytogenes. Amoxicillin does not reach adequate
concentrations in the central nervous system and is not appropriate for
meningitis therapy. Other indications for ampicillin in-clude serious
infections like enterococcal endocarditis and pneumonia caused by β-lactamase-negative
H. in-fluenzae. Amoxicillin oral
therapy is appropriate for clinically
acute nonserious bacterial infections like otitis media and sinusitis.
Amoxicillin also has use in mul-tidrug regimens for the eradication of Helicobacter py-lori in duodenal and
gastric ulcers.
Antipseudomonal Penicillins
Mezlocillin, piperacillin,
and ticarcillin are parenteral antibiotics formulated as sodium salts, so
prescribers must consider the sodium content of these antibiotics when
administering them to patients with congestive heart failure. During their
distribution phase, an-tipseudomonal penicillins achieve only low
concentra-tions in the cerebrospinal fluid. Consequently, an-tipseudomonal
penicillins are not among the drugs of first choice for meningitis therapy.
The antipseudomonal
penicillins undergo renal elimination (Table 45.1). Piperacillin and
ticarcillin have minimal hepatic metabolism. In contrast, me-zlocillin has
significant hepatic metabolism and requires dose adjustment in patients with
hepatic insufficiency.
The antipseudomonal
penicillins have comparable spectra of activity against many gram-positive and
gram-negative pathogens, including most anaerobes. Mezlocillin, piperacillin,
and ticarcillin have similar clin-ical outcomes in patients with known or
suspected P. aeruginosa infections. Antipseudomonal penicillins are used to treat pneumonias associated
with cystic fibrosis or mechanical ventilation.
Carbenicillin indanyl sodium
is an antipseudomonal penicillin formulated for oral administration. The drug
achieves negligible carbenicillin concentrations in the urine of patients with
renal failure. Consequently, car-benicillin is not appropriate for patients
with renal fail-ure. In patients with normal renal function, however,
carbenicillin indanyl sodium is used to treat urinary tract infections caused
by P. aeruginosa, Proteus spp., and Escherichia coli.
β-Lactamase Inhibitor Combinations
Several formulations combine
a β-lactam antibiotic with a β-lactamase inhibitor (ampicillin-sulbactam [Unasyn], ticarcillin-clavulanic acid [Timentin], piper-acillin-tazobactam [Zosyn], and amoxicillin–clavulanic acid
[Augmentin]). All of the β-lactamase
inhibitor combinations except amoxicillin-clavulanic acid are parenteral
formulations. Amoxicillin–clavulanic acid is the only combination drug with
oral bioavailability. Elimination of the combination drugs occurs primarily by
renal excretion. Therefore, all of the β-lactamase in-hibitor combinations
require dose adjustments in pa-tients with renal insufficiency. The addition of
the β-lactamase inhibitor significantly broadens the spectrum of antibacterial
activity against β-lactamase-producing organisms. Consequently, these drugs
have clinical use in treating infections with known or suspected mixed
bacterial flora, such as biliary infections, diabetic foot ulcers,
endomyometritis, and peritonitis.
β-Lactam Antibiotics in Pregnancy
All of the penicillin
antibiotics are classified by the U. S. Food and Drug Administration (FDA) in
pregnancy category B, that is, as drugs having either no fetal risk in animal
studies but human trials are inadequate, or animal studies show adverse fetal
effects but well-controlled human trials reveal no fetal damage. Obstetricians
frequently prescribe ampicillin, penicillin G, and penicillin V because they
are effective against the infections most frequently encountered in caring for
pregnant women (e.g., upper respiratory and lower uri-nary tract infections).
Adverse Effects
While being associated with a
low percentage of ad-verse reactions, the β-lactams are the most frequent source
of troublesome allergic reactions among the an-tibiotics. The overall frequency
of adverse effects asso-ciated with penicillin use is less than 10%, including
al-lergic and other reactions. Anaphylaxis is a serious, rare allergic response
with an occurrence rate between 0.004% and 0.015% of penicillin courses.
Allergic reactions to penicillin are immediate immunoglobulin (Ig) E–mediated
type I immune responses. Symptoms and signs of IgE-mediated reactions may
include ur-ticaria, pruritus, bronchospasm, angioedema, laryngeal edema, and
hypotension. Late onset immune-mediated reactions to β-lactam antibiotics may
manifest as eosinophilia, hemolytic anemia, interstitial nephritis, or serum
sickness. In contrast to the rare allergic reactions, nonallergic β-lactam
rashes are common. For example, ampicillin is associated with nonurticarial rashes
in 5 to 10% of recipients.
The incidence of nonallergic
ampicillin eruptions is 40 to 100% in patients with concomitant Epstein-Barr
virus (mononucleosis), cytomegalovirus, acute lympho-cytic leukemia, lymphoma,
or reticulosarcoma. Non-allergic penicillin-associated rashes are
characteristi-cally morbilliform (symmetrical, erythematous, confluent,
maculopapular) eruptions on the extremities. The onset of typical nonallergic
eruptions is more than 72 hours after β-lactam exposure. The mechanism for the
nonurticarial ampicillin rash is not known and is not related to IgE or type I
hypersensitivity. Penicillin skin tests are not useful in the evaluation of
nonurticarial ampicillin rashes. Patients with a history of nonurticar-ial
ampicillin rashes may receive other β-lactam antibi-otics without greater risk
of subsequent serious allergic reactions.
Allergic cross-reactivity
between β-lactam antibi-otics is significant. The frequency of allergic
reactions to another β-lactam antibiotic is 5.6% among patients with a history
of IgE-mediated hypersensitivity to one β-lactam antibiotic plus positive
results from a peni-cillin skin test. In general, patients with a convincing
his-tory of type I reaction to one β-lactam antibiotic should avoid all other β-lactam
antibiotics except aztreonam. However, most patients give unreliable histories
of penicillin allergy because of confusion with nonallergic penicillin rashes.
Among patients who report penicillin allergies, 80 to 90% have negative results
from peni-cillin skin tests, and 98% tolerate subsequent β-lactam antibiotic
treatments. A careful history may discrimi-nate between nonallergic reactions
and true penicillin allergy and permit safe β-lactam therapy.
Related Topics