Chapter: Medical Immunology: IgE-Mediated (Immediate) Hypersensitivity
Pathogenesis - IgE Mediated (Immediate) Hypersensitivity
PATHOGENESIS
The pathogenesis of immediate hypersensitivity reactions involves a well-defined sequence of events:
1. Synthesis of specific IgE antibodies.
2. Binding of IgE antibodies to Fcε -I receptors on basophils and mast cells; once bound, IgE acts as an antigen receptor.
3. Cross-linking of receptor-bound IgE by a multivalent antigen initiates the release of preformed vasoactive compounds and the synthesis and later release of medi-ators of inflammation.
4. The preformed substances released by basophils and mast cells have significant effects on target tissues, such as smooth muscle, vascular endothelium, and mu-cous glands. They also act as chemoattractant cytokines and may elicit central nervous system–mediated reflexes (e.g., sneezing).
5. Furthermore, activated basophils and mast cells can synthesize and express IL-4 and the CD40 ligand, both essential factors to stimulate IgE synthesis.
A. IgE Antibodies
Prausnitz and Küstner published the first demonstration that serum contains a factor capa-ble of mediating specific allergic reactions in 1921. The injection of serum from a fish-al-lergic person (Küstner) into Dr. Prausnitz’s skin and subsequent exposure of Dr. Prausnitz to fish antigen injected in the same site resulted in an allergic wheal and flare response.
In 1967 Ishizaka and collaborators isolated a new class of immunoglobulin, desig-nated as IgE, from the serum of ragweed-allergic individuals. Several patients with IgE-producing plasmocytomas were subsequently discovered and provided a source of very large amounts of monoclonal IgE that greatly facilitated further studies of IgE structure and the production of anti-IgE antibodies.
1. Quantitative Assay of IgE Antibodies
The total IgE concentration, even in allergic individuals, is extremely low, not detectable by most routine assays used for the assay of IgG, IgA, and IgM. The concentration of spe-cific IgE antibody to any given allergen is a very small fraction of the total IgE.
Early attempts to measure IgE involved cumbersome and often unreliable bioassays. The availability of anti-IgE antibodies allowed the development of radioimmunoassays sufficiently sensitive to determine total serum IgE and IgE antibody levels accurately.
The paper disc radioimmunosorbent test (PRIST) was one of the first solid-phase ra-dioimmunoassays introduced in diagnostic medicine. This assay, dia-grammatically summarized in Figure 21.1, measures total serum IgE.
1. A serum sample is added to a small piece of adsorbent paper to which anti-IgE antibodies are covalently bound. The immobilized antibody captures IgE.
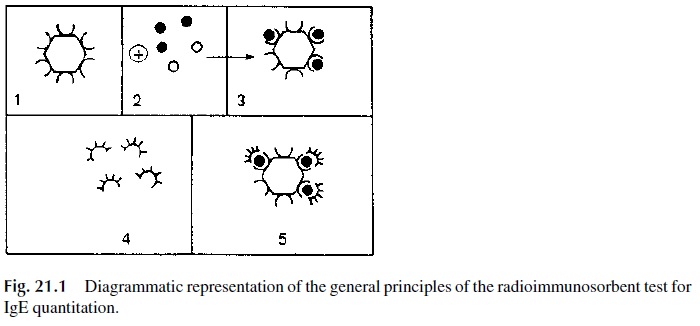
2. 125Iodine-labeled anti-IgE antibodies are subsequently added and will bind to the paper-bound-IgE. The radioactivity counted in the solid phase is directly related to the IgE level in the serum tested.
3. The results are expressed in nanograms/mL (1 ng = 10-6 mg) or in international units (1 I.U. = 2.5 ng/mL): 180 IU/mL is considered as the upper limit for nor-mal adults. Allergic individuals often have elevated levels of IgE. However, some asymptomatic individuals may also have elevated IgE levels. Therefore, a diagnosis of immediate hypersensitivity cannot be based solely on the determi-nation of abnormally elevated IgE levels.
Sensitive EIA and quantitative fluorescence assays were later developed that are equally able to measure total IgE levels without the need for use of radiolabeled com-pounds.
The radioallergosorbent test (RAST), diagrammatically summarized in Figure 21.2, is a solid phase radioimmunoassay that determines antigen-specific IgE, which from the di-agnostic point of view is considerably more relevant than the measurement of total serum IgE levels.
1. A given allergen (ragweed antigen, penicillin, β-lactoglobulin, etc.) is covalently bound to polydextran beads
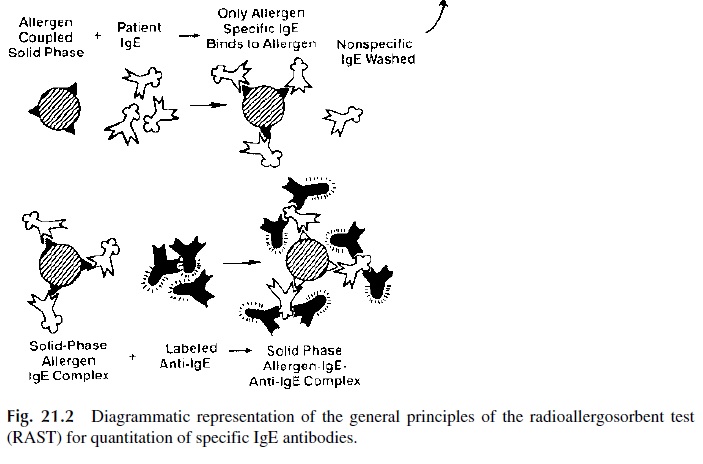
2. Patient’s serum is added to beads coated with a single antigen; the antigen-spe-cific IgE, if present, will bind to the immobilized antigen.
3. After washing off unbound immunoglobulins, radiolabeled anti-IgE is added. The amount of bead-bound radioactivity counted after washing off unbound la-beled antibody is directly related to the concentration of antigen-specific IgE present in the serum.
2. Skin Tests
Although the RAST assays are highly specific and accurate, they are expensive and lack sensitivity, and the range of antigens for which there are available tests is limited. In addi-tion, some authors have cast doubts about the biological relevance of the RAST assay re-sults. The alternative method for diagnosis of specific allergies is provocation skin tests, which allow testing to a wider array of antigens. Although positive skin tests depend on the existence of IgE antibodies, they do not allow a direct quantitative assay of such antibod-ies; rather, they provide information about their ability to mediate the hypersensitivity re-action. This explains the opinion of many specialists that the results of skin tests correlate better with clinical data than the results of the RAST assays.
The skin tests for immediate hypersensitivity are performed by injecting small amounts of purified allergens percutaneously or intradermally in known patterns. The pa-tients are then observed for about 30 minutes to one hour. Classical IgE-mediated hyper-sensitivity reactions present as a wheal and flare at the site of the allergen exposure, which develops in a matter of minutes.
In highly sensitized individuals, there is always a risk of anaphylaxis, even after min-imal challenge. Because of this risk, trained professionals should always perform these tests in a property equipped clinical facility.
B. The IgE Antibody Response
IgE is predominantly synthesized in perimucosal lymphoid tissues of the respiratory and gastrointestinal tract. In developing countries the main antigenic stimulus for IgE synthe-sis are parasites (particularly nematodes). Levels of circulating IgE considered as normal in a developing country with endemic parasitism are two to three orders of magnitude higher than in the western world. The vast majority of allergens, which are either ingested or inhaled, stimulate the same perimucosal tissues. In the perimucosal tissues only B lym-phocytes with membrane IgE will differentiate into IgE-producing plasma cells. Those IgE-carrying B cells are a only a small fraction of the total B cell population in the submu-cosa but are overrepresented in the perimucosal lymphoid tissues compared to other lym-phoid territories.
During the primary immune response to an allergen or a parasite, most of the IgE syn-thesized appears to be of low affinity. The changes occurring after a second exposure in-clude the synthesis of IgE of progressively higher affinity, probably as a consequence of somatic hypermutations. This may be the reason why allergic re-actions very seldom develop after the first exposure to an allergen. When a hypersensitiv-ity reaction appears to develop after what seems to be a first exposure to any given aller-gen, one must consider the possibility of cross-reaction between a substance to which the individual was previously sensitized and the substance that elicits the allergic reaction. Such cross-reactions are usually due to molecular mimicry and can be quite unpredictable.
Repeated exposures to parasites or allergens will stimulate the differentiation of memory cells and the proportion of circulating high-affinity antigen-specific IgE will also increase with repeated exposures. In patients suffering from severe pollen allergies, anti-gen-specific IgE may constitute up to 50% of the total IgE.
C. Genetic Control of IgE Synthesis
The study of total IgE levels in normal nonallergic individuals shows a distribution in three groups: high, intermediate, and low producers (Fig. 21.3). Family studies further suggested that the ability to produce high levels of IgE is a recessive trait, controlled by unknown genes independent of the HLA system. A candidate gene has been localized to chromosome 5(5q31–q33)—in close proximity to the genes for IL-4, IL-5, and IL-13. The gene in ques-tion seems not only to influence the synthesis of IgE, but also determines bronchial hyper-responsiveness to histamine and other mediators.
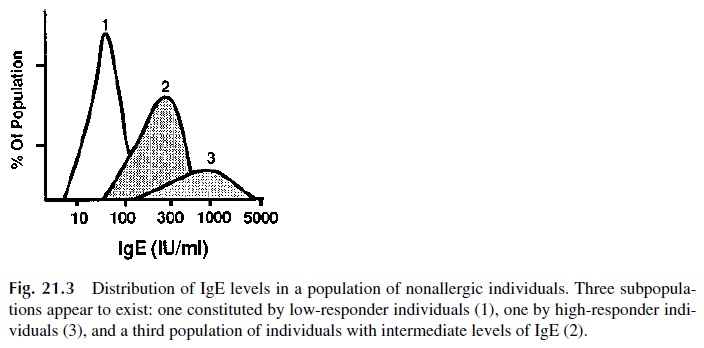
On the other hand, the tendency to develop allergic disorders in response to specific allergens is HLA linked. For instance, the ability to produce antigen-specific IgE after ex-posure to the Ra5 antigen of ragweed is observed more often in HLA B7, DR2 individuals than in the general population.
Recent studies suggest that various MHC class II antigens are associated with high responses to many different allergens (Fig. 21.4). The biological basis for the association between MHC-II molecules and allergic predisposition is believed to be one of the many expressions of the control exerted by APC on the immune response. This theory is based on the assumption that the MHC-II repertoire determines what anti-gen-derived peptides are most efficiently presented to helper T cells.
The genes controlling high IgE levels and high IgE antibody synthesis after exposure to allergens appear to have synergistic effects. For example, an HLA B7, DR2 individual who is also genetically predisposed to produce high levels of IgE is likely to have a more severe allergic disorder than an individual without this genetic combination.
D. T-B Cell Cooperation in IgE Antibody Responses
The role of activated TH2 cells in type I hypersensitivity reaction has been emphasized in recent years. Production of high levels of IgE has been shown to be dependent on the activation of IL-4–producing TH 2 cells as well as on the delivery of co-activating signals in-volving CD28/CD80 and CD40/CD40L. IL-4 has been clearly demonstrated to promote IgE synthesis by activated B lymphocytes. In addition, IL-4 is both a growth and differen-tiation factor for mast cells and basophils, causing their number to increase in all tissues where they will be able to bind more IgE. IL-5, which is produced at the same time by the same activated CD4 lymphocytes, is believed to play a role in the late phase of allergic re-actions.
Both in humans and animal models the production of allergen-specific IgE in humans persists long after the second exposure. This may result from the capture of IgE-containing immune complexes by dendritic cells, which express the Fcε -RII (CD23). Membrane-bound immune complexes are known to persist for longer periods of time, constantly stim-ulating B cells and promoting the persistence of secondary immune responses and the dif-ferentiation of antibodies with increased affinity . At the same time, other immune complexes, perhaps containing IgG antibody, will be internalized, processed, and be the source of allergen-derived peptides that will continue to activate the TH 2 subpopu-lation.
E. Interaction of IgE with Cell Surface Receptors
Two types of Fc receptors reacting with IgE molecules have been characterized.
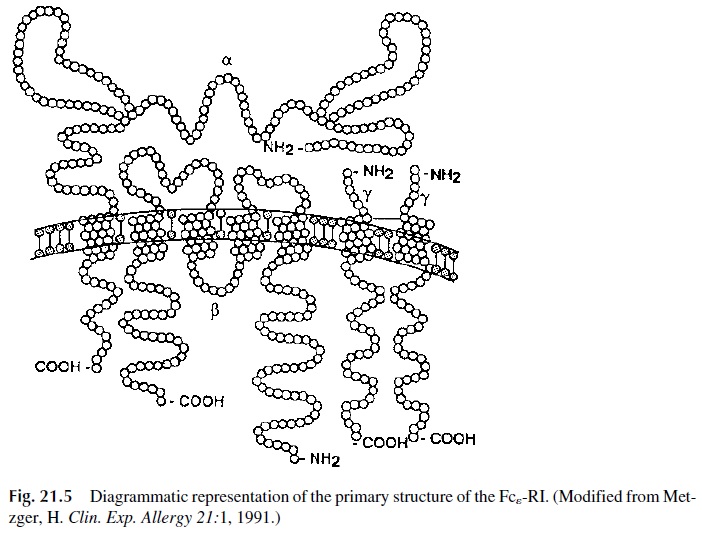
A unique high-affinity receptor designated as Fcε -RI, expressed on the surface of ba-sophils and mast cells. Most IgE antibodies interact with this receptor and become cell-as-sociated soon after secretion from plasma cells.
The structure of the Fcε -RI is unique among the well-characterized lymphoid cell re-ceptors. It is composed of three subunits: a heterodimer formed by the interaction of two chains (α and β), and a homodimer of a third type of chain (γ chain). The whole molecule is therefore designated as αβγ 2 (Fig. 21.5). The external domain of the α chain binds the Fc portion of IgE. The β and γ chains function as signal transduction units. Both of them contain ITAM motifs . Cross-linking of the Fcε -RI results in activation of the protein kinase Lyn, which phosphorylates ITAM tyrosines, leading then to the activa-tion of other protein kinases, such as Syk, as well as of the integral membrane linker molecule LAT . The phosphorylation of Syk and LAT is followed by ac-tivation of signaling cascades, which eventually lead to the release of performed granules and to the expression of a variety of genes coding for cytokines, enzymes, etc.
The interaction between the Fcε -RI and IgE is consistent with a simple bimolecular forward reaction and a first-order reverse reaction:
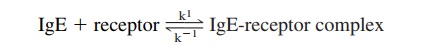
The affinity constant of the interaction, KA=k1/k-1 ranges from 108 to 1010 M/L -1. Because of the high affinity of the interaction between IgE and this Fcε -RI, IgE binds rapidly and very strongly to cells expressing it and is released from these cells very slowly. Passively transferred IgE remains cell-bound for several weeks in the skin of normal hu-mans. Because the mast cells and basophils do not produce IgE molecules, there is no clonal restriction at the mast cell/basophil level. Therefore, if the patient produces IgE an-tibodies to more than one allergen, each basophil or mast cell may bind IgE antibodies of different specificities.
The interaction between IgE and Fcε -RI does not result in cell activation. IgE serves as an antigen-receptor for mast cells and basophils. Receptor-bound IgE discriminates
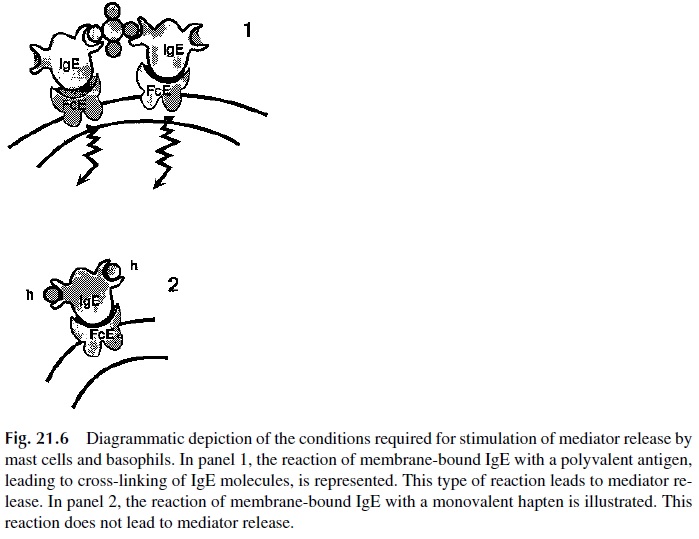
Re-ceptor-bound IgE must be cross-linked in order for basophils and mast cells to release their intracellular mediators (Fig. 21.6). The physiological cross-linking agent is the allergen, which is multivalent. Cross-linking of receptor bound IgE can also be induced with anti-IgE antibodies or with their divalent F(ab')2 fragments. Unoccupied receptors may be cross-linked with aggregated Fc fragments of IgE. In contrast, mast cells and basophils with IgE-antihapten antibodies on their membranes, cannot be stimulated by soluble, univalent, haptens, because those are unable to cross-link membrane IgE molecules. Stimulation is only possible when carrier-bound haptens are used, because those can cross-link many IgE molecules.
All the types of cross-linking listed above are equally efficient in activating IgE-car-rying mast cells and basophils. The details concerning the sequence of events in the ensu-ing activation cascade are still under investigation. The consequences of activation, how-ever, are well known: release of granule contents into the extracellular space and activation of the synthesis of additional mediators.
Cross-linking of receptor-bound IgE is not the only signal leading to the liberation of mediators from basophils and mast cells; these cells also respond to C3a, C5a, basic lyso-somal proteins, kinins, and autoantibodies of the IgG isotype directed against the subunit of the Fcε -RI (these autoantibodies are detected in 40% of the patients with chronic idio-pathic urticaria). It is apparent that there are multiple pathways for mast cell activation and that the participation of cell-bound IgE is not always needed.
Fcε -RII (CD23) is expressed on the membrane of lymphocytes, platelets, eosinophils, and dendritic cells and binds IgE with lower affinity than Fcε -RI. The role of Fcε -RII on dendritic cells has been previously discussed, and it is supposed to be involved in targeting eosinophils to parasites in one of the different variations of ADCC; its effect on platelets and lymphocytes is unclear.
F. Early and Late Phases in Type I Hypersensitivity
1. Early Phase
The metachromatic cytoplasmic granules of basophils and mast cells contain a variety of preformed mediators (Table 21.3). After cross-linking of Fc-receptor associated IgE, mast cells and basophils undergo a series of biochemical and structural changes.
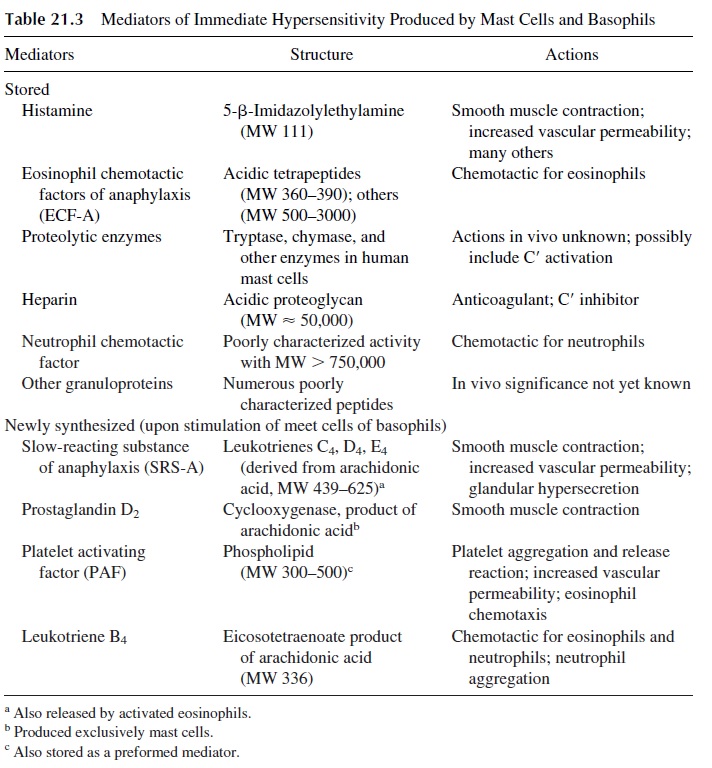
The first change to be detected is the polymerization of microtubules, which is en-ergy-dependent (inhibited by 2-deoxyglucose), enhanced by the addition of 3’ ,5’ -guano-sine monophosphate (GMP), and inhibited by the addition of 3’ ,5’ -adenosine monophos-phate (AMP) and colchicine. The polymerization of microtubules allows the transport of the cytoplasmic granules to the cell membrane to which they fuse. This is followed by the opening of the granules and the release of histamine and other preformed mediators, such as platelet-activating factor (PAF) and chemotactic factors for eosinophils (ECFA), into the surrounding medium. In vitro, this sequence of events takes 30–60 seconds.
Histamine is the mediator responsible for most of the symptoms observed during the early phase of allergic reactions. Since the constricting effect of histamine on the smooth muscle lasts only 1 or 2 hours, this phase stops shortly after most of the granules have been emptied.
Platelet-activating factor (PAF), a mediator rapidly released after basophil/mast cell activation, is a phospholipid whose effects include platelet aggregation, chemotaxis of eosinophils, release of vasoactive amines, and increased vascular permeability (both due to a direct effect and to the release of vasoactive amines). PAF is also released by neutrophils and other cells.
2. Late Phase
The cells remain viable after degranulation and proceed to synthesize other substances, which will be released at a later time, causing the late phase of a type I hypersensitivity reaction. The mediators responsible for the late phase of the response are not detected until several hours after release of histamine and other preformed mediators. The long la-tency period between cell stimulation and detection of these mediators suggest that they are synthesized by mast cells after stimulation and/or by cells attracted by the above-mentioned chemotactic factors. The main mediators involved in the late phase are leukotrienes C4, D4, and E4 [LTC4, LTD4, LTE4, slow reacting substance of anaphylaxis (SRS-A)]. This mixture of leukotrienes previously known as SRS-A reaches effective con-centrations only 5–6 hours after challenge and have effects on target cells lasting for sev-eral hours. LTC4 and LTD4are several fold more potent than histamine in causing smooth muscle contraction, bronchovascular leak, and mucous hypersecretion in human bronchi.
3. Role of Eosinophils in the Late Phase
Eosinophils are attracted to the site where an immediate hypersensitivity reaction is taking place by chemotactic factors released by basophils, mast cells, and TH 2 lymphocytes:
· ECFA and PAF, preformed chemotactic factors released during basophil or mast cell degranulation
· Leukotriene B4, synthesized and released by stimulated basophils/mast cells Interleukin-5, released by activated TH 2 lymphocytes, mast cells, and eosinophils
In many cases, the appearance of eosinophils signals the onset of internal negative feedback and control mechanisms that terminate the immediate hypersensitivity reaction. This effect is associated with the production and release of enzymes, particularly histami-nase (which degrades histamine) and phospholipase D (which degrades PAF). Active oxy-gen radicals released by stimulated granulocytes, including eosinophils and perhaps neu-trophils (which are also attracted by ECF-A and LTB4), cause the breakdown of leukotrienes. Histamine itself can contribute to the downregulation of the allergic reaction by binding to a type II histamine receptor expressed on basophils and mast cells; the occu-pancy of this receptor leads to an intracellular increase in the level of cAMP which inhibits further release of histamine (negative feedback).
In contrast, persistent eosinophil infiltrates are associated with intense inflammation that causes prolongation of symptoms. For example, asthmatic patients may develop a pro-longed crisis during which the symptoms remain severe, and breathing becomes progres-sively more difficult, leading to a situation of increasing respiratory distress, which does not respond to the usual treatment.
In these patients it is frequent to find very heavy peribronchial cellular infiltrates of the epithelium and lamina propria, where eosinophils predominate, but containing also T lymphocytes and plasma cells (chronic eosinophilic bronchitis). The infiltrating lympho-cytes are mostly activated CD4, CD25+ T lymphocytes, whose cytokine mRNA pattern is typical of the TH 2 subpopulation.
Three major cytokines released by TH 2 cells are believed t play significant roles in immediate hypersensitivity reactions, particularly in bronchial asthma:
1. IL-4 is a critical factor in promoting TH 2-cell differentiation, B-cell activation, and switch to IgE synthesis
2. IL-13, a cytokine related to IL-4, has been shown in experimental animal mod-els to induce the pathophysiological features of asthma in an IgE and eosinophil-independent manner. This observations raise the interesting possibility that asthma may be mediated not only by IgE-producing B cells, but also by activated TH 2 cells.
3. IL-5 released from the infiltrating activated TH 2 lymphocytes seems to attract, retain, and activate eosinophils. Activated eosinophils release leukotrienes and two toxic proteins: eosinophilic cationic protein and major basic protein. These proteins decrease cilliary movement, are cytotoxic for bronchial epithelium, and cause nonspecific bronchial hyperactivity and cellular denudation. This is be-lieved to be a critical step in the pathogenesis of chronic airways inflammation that after decades may lead to chronic obstructive pulmonary disease with irre-versible remodeling of the airways.
A similar pathogenic sequence seems responsible for a state of bronchial hyperresponsiveness to many stimuli that develops in the wake of an asthma crisis.
The severity of the clinical symptoms in an immediate hypersensitivity reaction is di-rectly related to the amount of mediators released and produced, which in turn is deter-mined by the number of “sensitized” cells stimulated by the antigen. The expression of early and late phases can be distinguished clinically:
· In the case of a positive immediate reaction elicited by a skin test, the early phase re-solves in 30–60 minutes; the late phase generally peaks at 6–8 hours and re-solves at 24 hours.
· In the case of an asthma crisis, the early phase is characterized by shortness of breath and nonproductive cough and lasts 4–5 hours. If the exposure to the responsi-ble airborne allergen is very intense, life-threatening bronchospasm may de-velop. With less intense or chronic exposure, the initial symptoms of wheezing and dry cough will linger for a few hours.
· Around the sixth hour, the cough starts to produce sputum, signaling the onset of the late phase during which the dyspnea may become more severe. In very severe cases, death may occur at this late stage because of persistent bronchoconstric-tion and peribronchial inflammation associated with increased mucous secre-tion, both factors contributing to severe airflow obstruction.
Related Topics