Chapter: Essentials of Anatomy and Physiology: The chemical basis of life
Organic Molecules
ORGANIC MOLECULES
A. Describe the structural organization and major functions ofcarbohydrates, lipids, proteins, and nucleic acids.
B. Explain how enzymes work
Carbon’s ability to form covalent bonds with other atoms makes possible the formation of the large, diverse, complicated molecules necessary for life. Carbon atoms bound together by covalent bondsconstitute the “framework” of many large molecules. Two mecha-nisms that allow the formation of a wide variety of molecules are variation in the length of the carbon chains and the combination of the atoms bound to the carbon framework. For example, proteins have thousands of carbon atoms bound by covalent bonds to one another and to other atoms, such as nitrogen, sulfur, hydrogen, and oxygen.
The four major groups of organic molecules essential to living organisms are carbohydrates, lipids, proteins, and nucleic acids. Each of these groups has specific structural and functional charac-teristics (table 2.4).
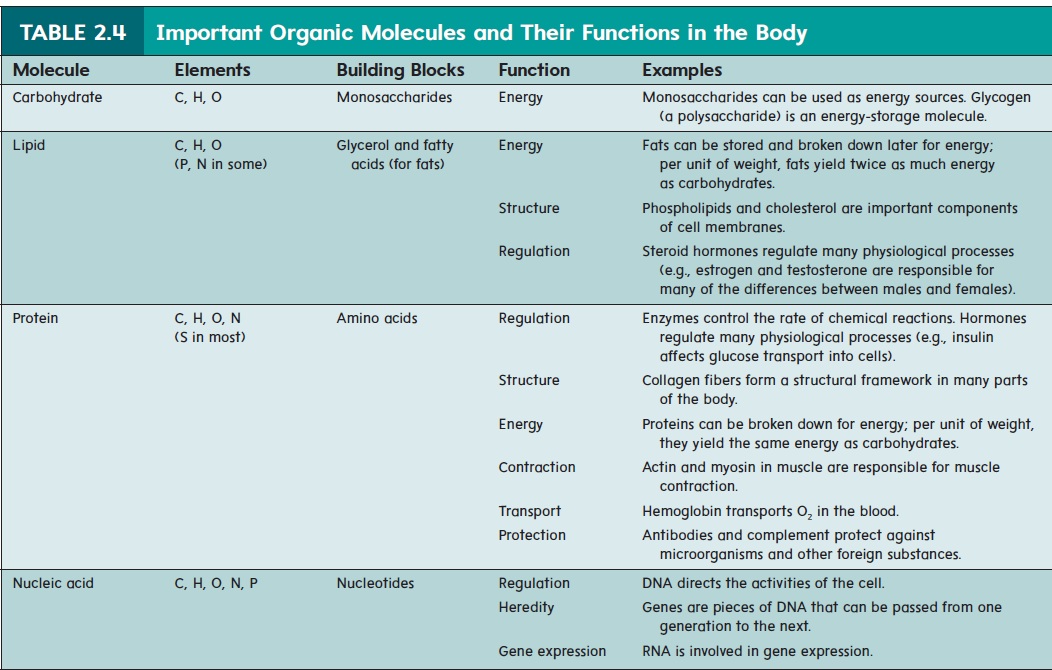
Carbohydrates
Carbohydrates are composed of carbon, hydrogen, and oxygenatoms. In most carbohydrates, for each carbon atom there are two hydrogen atoms and one oxygen atom. Note that this two-to-one ratio is the same as in water (H2O). The molecules are called carbo-hydrates because each carbon (carbo-) is combined with the same atoms that form water (hydrated). For example, the chemical formula for glucose is C6H12O6.
The smallest carbohydrates are monosaccharides (mon-ō-sak′\ă-rı̄dz; one sugar), or simple sugars. Glucose (blood sugar) and fructose (fruit sugar) are important monosaccharide energy sources for many of the body’s cells. Larger carbohydrates are formed by chemically binding monosaccharides together. For this reason, monosaccharides are considered the building blocks of carbohydrates.Disaccharides (dı̄-sak′\ă-rı̄dz; two sugars) are formed when two monosaccharides are joined by a covalent bond. For example, glucose and fructose combine to form the disac-charide sucrose (table sugar) (figure 2.11a). Polysaccharides (pol-ē-sak′\ă-rı̄dz; many sugars) consist of many monosaccharides bound in long chains. Glycogen, or animal starch, is a polysac-charide of glucose (figure 2.11b). When cells containing glycogen need energy, the glycogen is broken down into individual glucose molecules, which can be used as energy sources. Plant starch, also a polysaccharide of glucose, can be ingested and broken down into glucose. Cellulose, another polysaccharide of glucose, is an important structural component of plant cell walls. Humans cannot digest cellulose, however, and it is eliminated in the feces, where the cellulose\ fibers provide bulk.
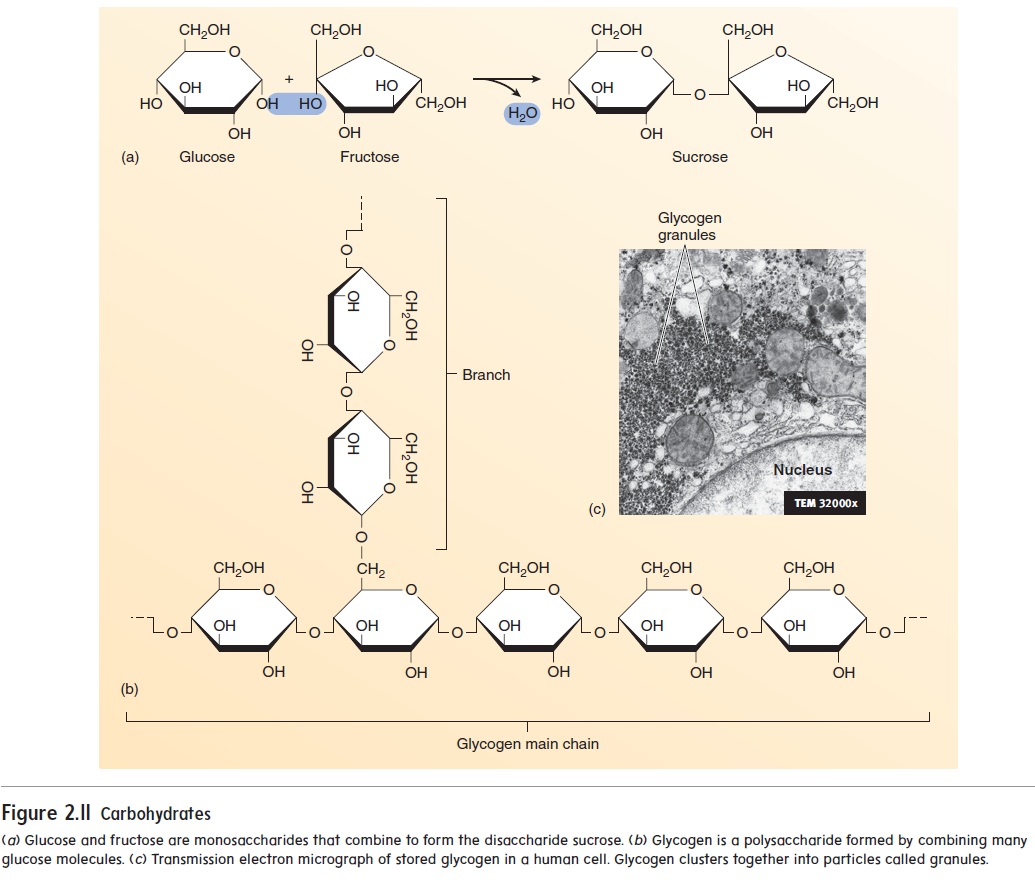
Lipids
Lipids are substances that dissolve in nonpolar solvents, such asalcohol or acetone, but not in polar solvents, such as water. Lipids are composed mainly of carbon, hydrogen, and oxygen, but other elements, such as phosphorus and nitrogen, are minor components of some lipids. Lipids contain a lower proportion of oxygen to carbon than do carbohydrates.
Fats, phospholipids, eicosanoids, and steroids are examples of lipids. Fats are important energy-storage molecules; they also pad and insulate the body. The building blocks of fats are glycerol (glis′\er-ol) and fatty acids (figure 2.12). Glycerol is a 3-carbon
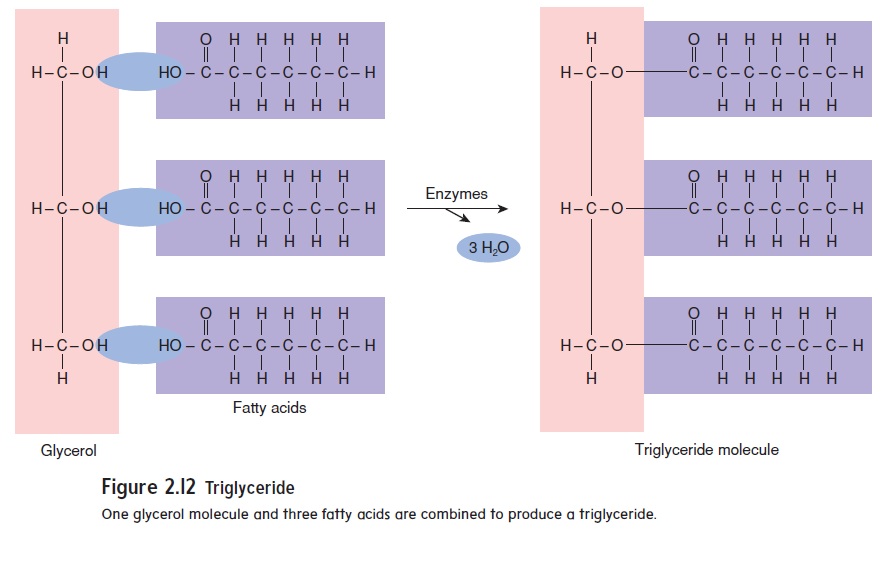
Figure 2.11 Carbohydrates
molecule with a hydroxyl (hı̄-drok′\sil) group (—OH) attached to each carbon atom, and fatty acids consist of a carbon chain with a carboxyl (kar-bok′\sil) group attached at one end. A car-boxyl group consists of both an oxygen atom and a hydroxyl group attached to a carbon atom (—COOH):
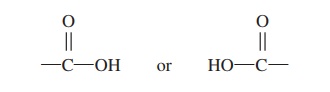
The carboxyl group is responsible for the acidic nature of the molecule because it releases H+ into solution. Triglycerides (trı̄-glis′\er-ı̄dz) are the most common type of fat molecules. Triglycerides have three fatty acids bound to a glycerol molecule.
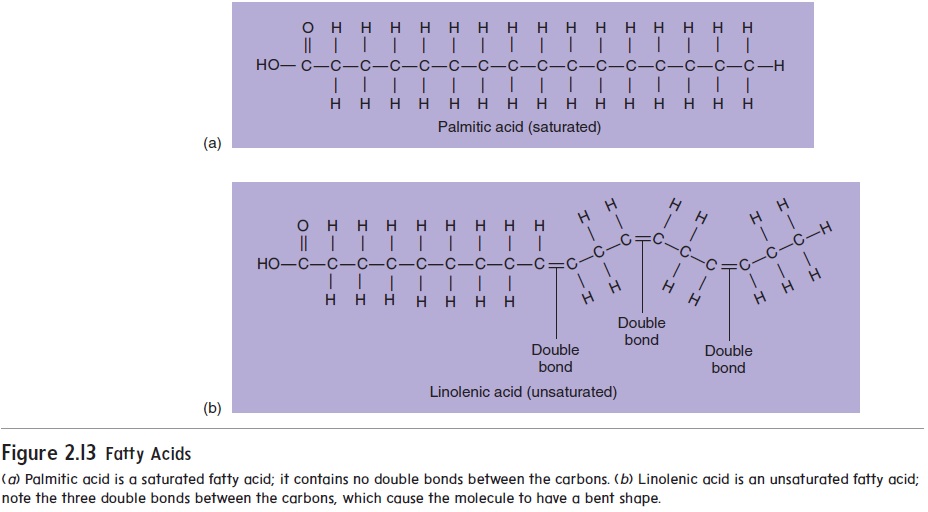
Fatty acids differ from one another according to the length and degree of saturation of their carbon chains. Most naturally occurring fatty acids contain 14 to 18 carbon atoms. A fatty acid is saturatedif it contains only single covalent bonds between the carbon atoms (figure 2.13a). Sources of saturated fats include beef, pork, whole milk, cheese, butter, eggs, coconut oil, and palm oil. The carbon chain isunsaturated if it has one or more double covalent bonds (figure 2.13b). Because the double covalent bonds can occur anywhere along the carbon chain, many types of unsaturated fatty acids with an equal degree of unsaturation are possible. Monounsaturated fats, such as olive and peanut oils, have one double covalent bond between carbon atoms. Polyunsaturated fats, such as safflower, sunflower, corn, and fish oils, have two or more double covalent bonds between carbon atoms. Unsaturated fats are the best type of fats in the diet because, unlike saturated fats, they do not contribute to the development of cardiovascular disease.
Transfats are unsaturated fats that have been chemically altered by the addition of H atoms. The process makes the fats more saturated and hence more solid and stable (longer shelf-life). However, the change in structure of these chemicals makes the consumption of trans fats an even greater factor than saturated fats in the risk for cardiovascular disease.
One glycerol molecule and three fatty acids are combined to produce a triglyceride.
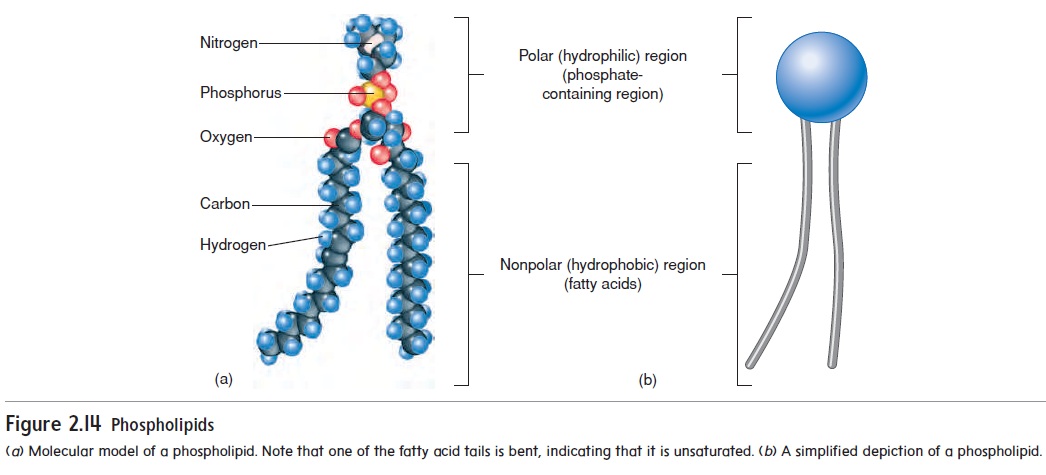
Phospholipids are similar to triglycerides, except that oneof the fatty acids bound to the glycerol is replaced by a molecule containing phosphorus (figure 2.14). A phospholipid is polar at the end of the molecule to whichthe phosphate is bound and nonpolar at the other end. The polar end of the molecule is attracted to water and is said to be hydrophilic (water-loving). The non\polar end is repelled by water and is said to be hydrophobic (water-fearing).Phospholipids are important structural components of cell mem-branes .
The eicosanoids (ı̄′\kō-să-noydz) are a group of important chemicals derived from fatty acids. Eicosanoids are made in most cells and are important regulatory molecules. Among their numer-ous effects is their role in the response of tissues to injuries. One example of eicosanoids is prostaglandins (pros′\tă-glan′\dinz).which have been implicated in regulating the secretion of some hormones, blood clotting, some reproductive functions, and many other processes. Many of the therapeutic effects of aspirin and other anti-inflammatory drugs result from their ability to inhibit prosta-glandin synthesis.
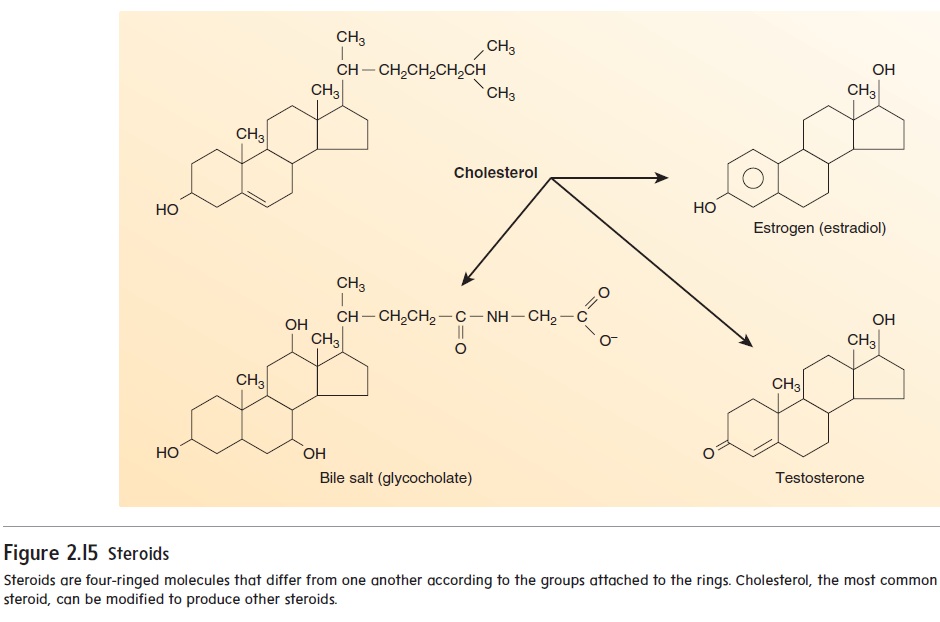
Steroids are composed of carbon atoms bound together into four ringlike structures. Cholesterol is an important steroid because other steroid molecules are synthesized from it. For example, bile salts, which increase fat absorption in the intestines, are derived from cholesterol, as are the reproductive hormones estrogen, pro-gesterone, and testosterone (figure 2.15). In addition, cholesterol is an important component of cell membranes. Although high levels of cholesterol in the blood increase the risk of cardiovascular disease, a certain amount of cholesterol is vital for normal function.
Proteins
All proteins contain carbon, hydrogen, oxygen, and nitrogen, and most have some sulfur. The building blocks of proteins are amino (ă-mē′\nō) acids, which are organic acids containing an amine (ă-mēn′\)group (–NH2) and a carboxyl group (figure 2.16a). There are 20 basic types of amino acids. Humans can synthesize 12 of them from simple organic molecules, but the remaining 8 so-called essential amino acids must be obtained in the diet.
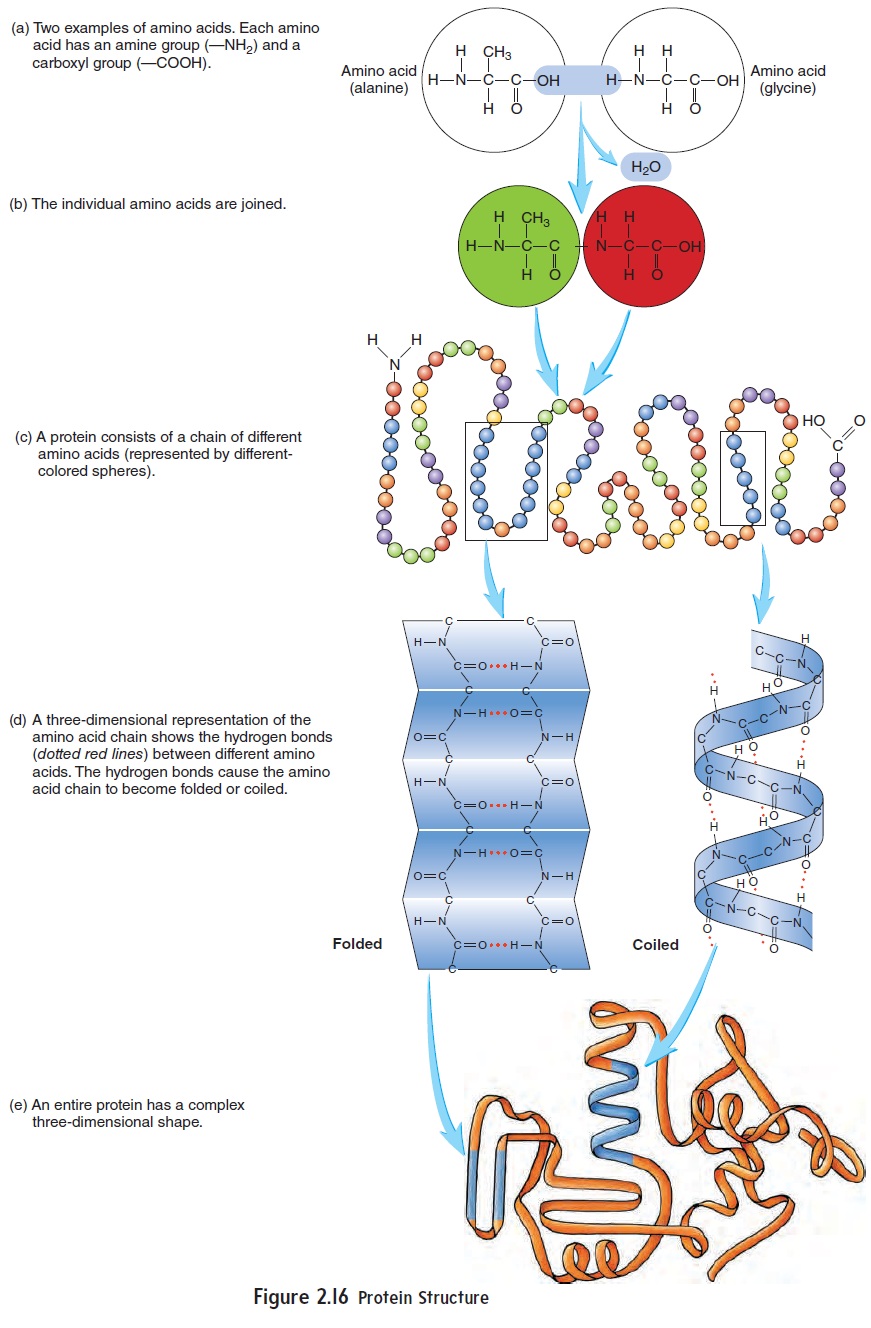
A protein consists of many amino acids joined together to form a chain (figure 2.16b,c). Although there are only 20 amino acids, they can combine to form numerous types of proteins with unique structures and functions. Different proteins have different kinds and numbers of amino acids. Hydrogen bonds between amino acids in the chain cause the chain to fold or coil into a specific three-dimensional shape (figure 2.16d,e). The ability of proteins to perform their functions depends on their shape. If the hydrogen bonds that maintain the shape of the protein are broken, the protein becomes nonfunctional. This change in shape is called denaturation, and it can be caused by abnormally high temperaturesor changes in pH.
Proteins perform many important functions. For example, enzymes are proteins that regulate the rate of chemical reactions, structural proteins provide the framework for many of the body’s tissues, and muscles contain proteins that are responsible for muscle contraction.
Enzymes
An enzyme (en′\zı̄m) is a protein catalyst that increases the rate at which a chemical reaction proceeds without the enzyme being permanently changed. Enzymes increase the rate of chemical \reactions by lowering the activation energy, which is the energy necessary to start a chemical reaction. For example, heat in the form of a spark is required to start the reaction between O2 and gasoline. Most of the chemical reactions that occur in the body have high activation energies, which are decreased by enzymes (figure 2.17). The lowered activation energies enable reactions to proceed at rates that sustain life.
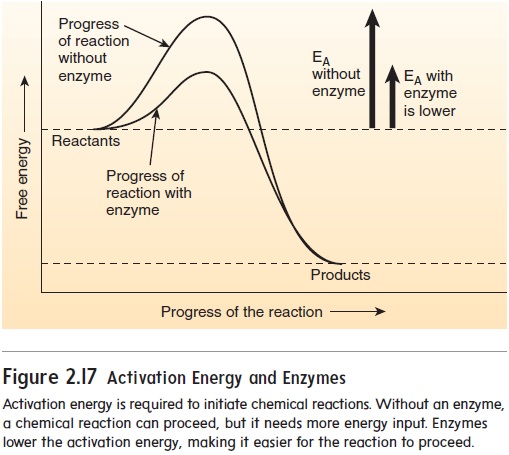
Consider an analogy in which paper clips represent amino acids and your hands represent enzymes. Paper clips in a box only occasionally join together. Using your hands, however, you can rapidly make a chain of paper clips. In a similar fashion, enzymes can quickly join amino acids into a chain, forming a protein. An enzyme allows the rate of a chemical reaction to take place more than a million times faster than it would without the enzyme.
The three-dimensional shape of enzymes is critical for their normal function. According to the lock-and-key model of enzymeaction, the shape of an enzyme and those of the reactants allow the enzyme to bind easily to the reactants. Bringing the reactants very close to one another reduces the activation energy for the reaction. Because the enzyme and the reactants must fit together, enzymes are very specific for the reactions they control, and each enzyme controls only one type of chemical reaction. After the reaction takes place, the enzyme is released and can be used again (figure 2.18).
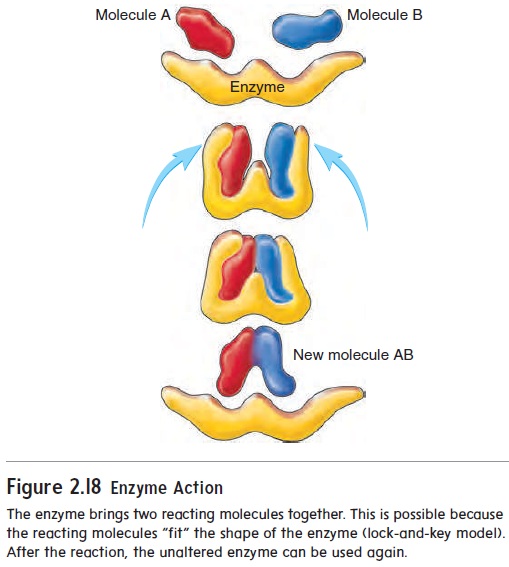
The body’s chemical events are regulated primarily by mechanisms that control either the concentration or the activity of enzymes. Either (1) the rate at which enzymes are produced in cells or (2) whether the enzymes are in an active or inactive form determines the rate of each chemical reaction.
Nucleic Acids: DNA and RNA
Deoxyribonucleic (dē̄-oks′\ē-rı̄′\bō-noo-klē′\ik) acid (DNA) is thegenetic material of cells, and copies of DNA are transferred from one generation of cells to the next. DNA contains the information that determines the structure of proteins. Ribonucleic (rı̄′\bō-noo-klē′\ik) acid (RNA) is structurally related to DNA, and three types of RNA also play important roles in gene expression or protein synthesis.
The nucleic (noo-klē′\ik, noo-klā′\ik) acids are large mol\ ecules composed of carbon, hydrogen, oxygen, nitrogen, and phosphorus. Both DNA and RNA consist of basic building blocks called nucleotides(noo′\klē-ō-t ı̄dz). Each nucleotide is composed of a sugar (monosaccharide) to which a nitrogenous organic base and a phosphate group are attached (figure 2.19). The sugar is deoxyribose for DNA, ribose for RNA. The organic bases are thy-mine (thı̄′\mēn, thı̄′\min), cytosine (sı̄′\tō-sēn), and uracil (ūr′\ă-sil), which are single-ringed molecules, and adenine (ad′\ĕ-nēn) and guanine (gwahn′\ēn), which are double-ringed molecules.
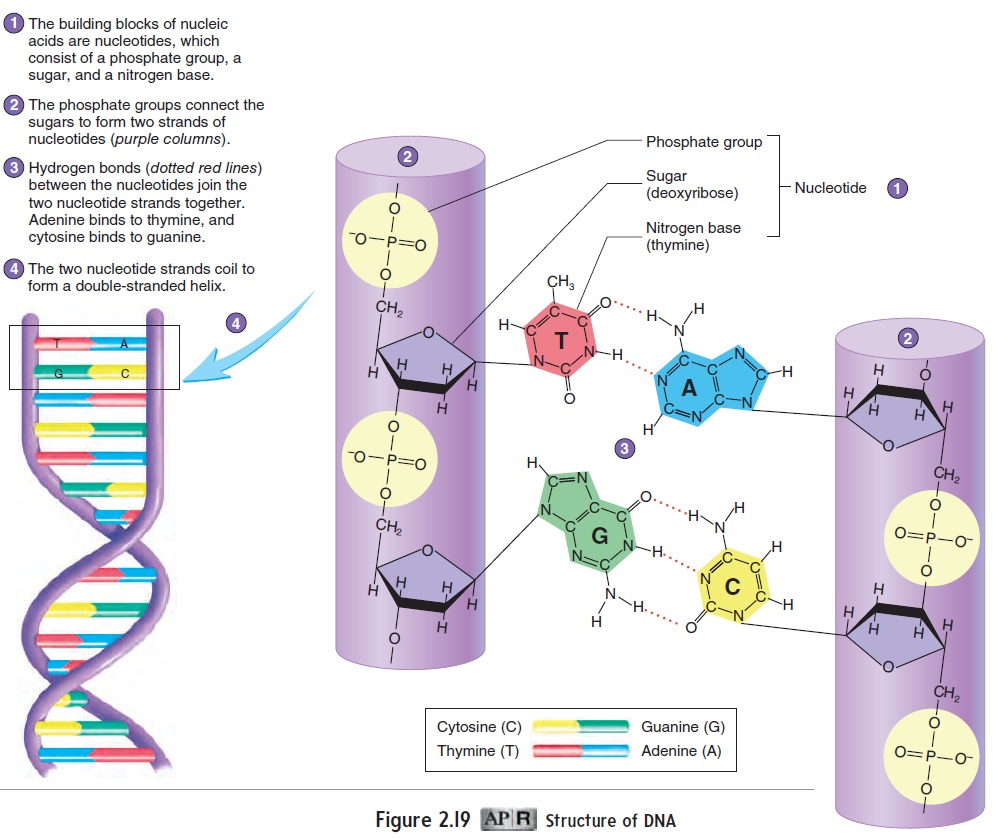
DNA has two strands of nucleotides joined together to form a twisted, ladderlike structure called a double helix. The sides of the ladder are formed by covalent bonds between the sugar molecules and phosphate groups of adjacent nucleotides. The rungs of the lad-der are formed by the bases of the nucleotides of one side connected to the bases of the other side by hydrogen bonds. Each nucleotide of DNA contains one of the organic bases: adenine, thymine, cytosine, or guanine. Adenine binds only to thymine because the structure of these organic bases allows two hydrogen bonds to form between them. Cytosine binds only to guanine because the structure of these organic bases allows three hydrogen bonds to form between them.
The sequence of organic bases in DNA molecules stores genetic information. Each DNA molecule consists of millions of organic bases, and their sequence ultimately determines the type and sequence of amino acids found in protein molecules. Because enzymes are proteins, DNA structure determines the rate and type of chemical reactions that occur in cells by controlling enzyme structure. Therefore, the information contained in DNA ultimately defines all cellular activities. Other proteins, such as collagen, that are coded by DNA determine many of the structural features of humans.
RNA has a structure similar to a single strand of DNA. Like DNA, four different nucleotides make up the RNA molecule, and the organic bases are the same, except that thymine is replaced with uracil. Uracil can bind only to adenine.
Adenosine Triphosphate
Adenosine triphosphate (ă-den′\ō-sēn trı̄-fos′\fāt) (ATP) is anespecially important organic molecule found in all living organisms. It consists of adenosine (the sugar ribose with the organic base ade-nine) and three phosphate groups (figure 2.20). Adenosine diphos-phate (ADP) has only two phosphate groups. The potential energy stored in the covalent bond between the second and third phosphate groups is important to living organisms because it \provides the energy used in nearly all of the chemical reactions within cells.
ATP is often called the energy currency of cells because it is capable of both storing and providing energy. The concentration of ATP is maintained within a narrow range of values, and essentially all energy-requiring chemical reactions stop when the quantity of ATP becomes inadequate.
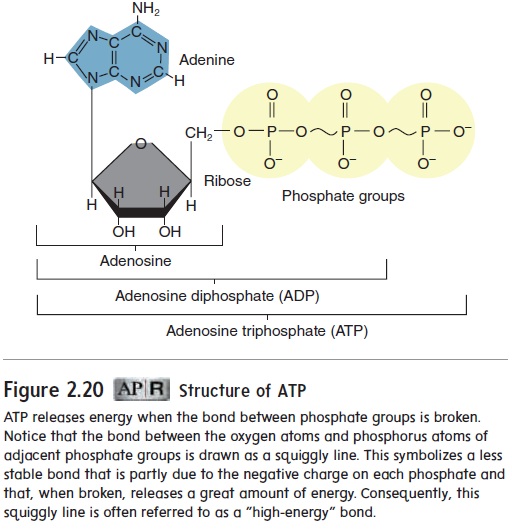
ATP releases energy when the bond between phosphate groups is broken. Notice that the bond between the oxygen atoms and phosphorus atoms of adjacent phosphate groups is drawn as a squiggly line. This symbolizes a less stable bond that is partly due to the negative charge on each phosphate and that, when broken, releases a great amount of energy. Consequently, this squiggly line is often referred to as a "high-energy" bond.
Related Topics