Chapter: Modern Analytical Chemistry: Chromatographic and Electrophoretic Methods
Optimizing Chromatographic Separations Using the Capacity Factor to Optimize Resolution
Using the Capacity Factor to Optimize Resolution
One of the simplest ways to improve resolution is to adjust the capacity factor for solute B. If all other terms in equation 12.21 remain constant, increasing kB’ improves resolution. As shown in Figure 12.11, however, the effect is greatest when the original capacity factor is small.
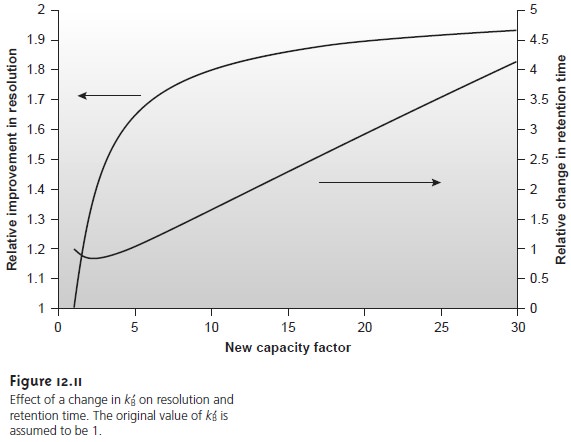
Furthermore, large increases in kB’ do not lead
to proportionally larger increases
in resolution. For example, when the original
value of kB’ is 1, increasing its value to 10 gives
an 82% improvement in resolution; a fur- ther increase to 15 provides a net improvement in resolution of only 87.5%.
Any improvement in resolution obtained by increasing kB’
generally comes at the expense of a longer
analysis time. This is also indicated in Figure 12.11,
which shows the relative
change in retention
time as a function of the new capacity factor. Note that a minimum
in the retention time curve occurs
when kB’ is equal to 2, and that retention time increases in either direction. Increasing kB’ from
2 to 10, for ex- ample, approximately doubles solute
B’s retention time.
The relationship between
capacity factor and
analysis time can
be advantageous when a separation produces
an acceptable resolution with a large kB’. In this case it
may be possible to decrease kB’ with little
loss in resolution while significantly short- ening the analysis time.
A solute’s capacity
factor is directly
proportional to its distribution ratio (equa-
tion 12.6), which, in turn,
is proportional to the solute’s equilibrium distribution
constant. To increase kB’ without
significantly changing α, which also is a function
of kB’, it is necessary to alter chromatographic conditions in a way that leads to a
general, nonselective increase
in the capacity factor for both solutes.
In gas chro- matography, this is usually accomplished by decreasing the
column’s temperature. At a lower temperature a solute’s vapor
pressure decreases, ensuring
that it spends more time in the stationary phase
increasing its capacity
factor. In liquid
chro- matography, changing the mobile phase’s
solvent strength is the easiest
way to change a solute’s capacity
factor. When the mobile phase
has a low solvent strength, solutes spend proportionally more time in the stationary phase, thereby increasing their capacity factors. Additionally, equation 12.6 shows
that the capacity
factor is proportional to the volume
of stationary phase.
Increasing the volume
of stationary phase, therefore, also leads to an increase
in kB’.
Adjusting the capacity factor to improve resolution between one pair of solutes may lead to an unacceptably long retention time for other solutes.
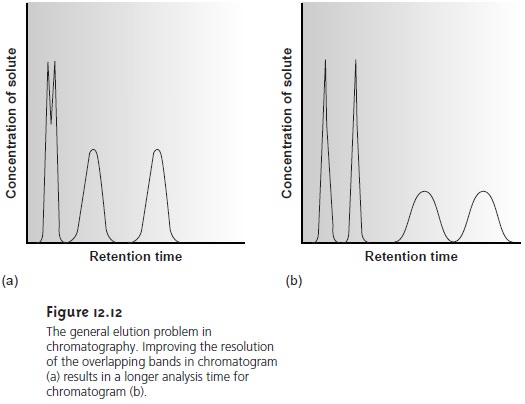
For ex- ample, improving resolution for solutes with short retention times by increasing on the
other hand, decreasing kB’ as a means
of shortening the
overall analysis time
may lead to a loss of resolution for solutes eluting
with shorter retention times. This difficulty
is encountered so frequently that it is known as the general elution
problem (Figure 12.12).
One solution to the general
elution problem is to make incremental adjustments to the
capacity factor over time. Thus, initial chromato-
graphic conditions are adjusted to enhance the resolution for solutes with short
retention times. As the separation progresses, chromatographic conditions are changed
in a manner that increases
the elution rate (decreases the retention time) for
later eluting solutes. In gas chromatography this is accomplished by tempera- ture programming.
The column’s initial
temperature is selected
such that the first solutes
to elute are fully resolved. The temperature is then increased, either continuously or in steps, to bring off later eluting
components with both an ac- ceptable resolution and a reasonable analysis
time. In liquid chromatography the
same effect can be obtained by increasing the
solvent’s eluting strength. This is known as a gradient
elution.
Related Topics