Chapter: Pharmaceutical Drug Analysis: Non-Aqueous Titrations
Non-Aqueous Titrations: Theory
THEORY
The concepts of the Lowry-Bronsted theory may explain the
various reactions that take place during many non-aqueous titrations. Thus, an acid is a proton donor and a base
is a proton acceptor. Therefore,
when an acid HA undergoes dissociation it gives rise to a proton and the
conjugate base A of the acid :

In other words, the liberated base A shall unite with a
proton to give the corresponding conjugate acid HA of the base A because every
base has its conjugate acid and vice
versa.
Hence, from the above definitions it may be implied that
:
(a) an acid : could be either an
electrically neutral molecule e.g.,
HNO3 ; or a negatively charged anion e.g., HSO4– ; or a positively charged cation e.g., C6H5NH2+
, H3O ;
(b) a base : could be either an
electrically neutral molecule e.g., C6H5NH2
; or an anion e.g., Cl–,
NO3–.
1. SOLVENTS
These are of three
types and they will be discussed briefly here :
(a) Protophillic Solvents : They are
essentially basic in nature and normally react with acids to form solvated
protons :
Example :

Perchloric acid displays more strongly acidic
characteristics than a weak acid, for instance : acetic acid when dissolved in
a weakly basic solvent.
(b) Protogenic Solvents : They are acidic
in nature and character e.g.,
sulphuric acid. They exert a ‘levelling
effect’ on bases i.e., they
become indistinguishable in strength when dissolved in strongly basic solvents
due to their enhanced affinity of strong bases for protons.
(c) Amphiprotic Solvents : They possess
both protophillic and protogenic characteristics.
Examples : Acetic acid, water and
alcohols.
They undergo dissociation to a very less extent. Acetic
acid is mostly employed as a solvent for the titration of basic substances and
its dissociation can be depicted as shown below :
CH3COOH
< == > H+
+ CH3COO–
In the above instance acetic acid is behaving as an acid.
Perchloric Acid : It is a very strong acid and
when it is made to dissolve in acetic acid, the latter can behave as a base and forms an ‘onium ion’ after combining with protons donated by the perchloric
acid. Thus, we have :
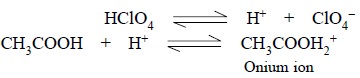
As the CH3COOH2+ ion can
instantly donate its proton to a base, therefore, a solution of perchloric acid
in glacial acetic acid, behaves as a strongly acidic solution.
Pyridine, a weak base, when dissolved
in acetic acid, the latter exerts its levelling effect and subsequently increases the basic characteristics of the
pyridine. Therefore, it is practically feasible to titrate a solution of a weak
base in acetic acid against a mixture of perchloric acid in acetic acid. Thus,
a sharp end point is achieved which otherwise cannot be obtained when the
titration is performed in an aqueous medium.
The various reactions with perchloric acid, acetic acid
and pyridine are summarized below :
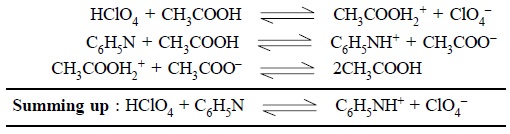
Acetonitrile, acetone and dimethylformamide—these
non-aqueous solvents exert a greater differen-tial in the protophillic
properties of many substances than in the corresponding aqueous solutions, due
to the levelling effect of water in the latter solutions. Hence, the most
acidic substance in aqueous solutions of a number of acids is the formation of
the hydronium ion as shown below :
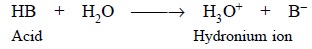
It is pertinent to observe here that the following
inorganic acids almost exhibit equal strength in aqueous solutions, whereas in
non-aqueous solvents, their ‘acidity’
retards in the following order :
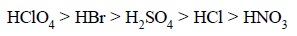
In glacial acetic acid (an acidic solvent) and in dioxane
(a neutral solvent), the perchloric acid (HClO4) behaves as more
acidic (i.e., less protophyllic) than
HCl; and, therefore, many base-hydrochlorides (i.e., chlorides) may be titrated with standard HClO4,
just as carbonates may be titrated in aqueous solution with standard HCl.
In short, it is possible to titrate mixtures of two or
three components selectively with a single titration by wisdom of the right
choice of solvent for the non-aqueous titrations.
Related Topics