Chapter: 11th Chemistry : UNIT 13 : Hydrocarbons
Nomenclature and Isomerism of Aromatic Hydrocarbons
Nomenclature and Isomerism
• We have already discussed about nomenclature of aromatic hydrocarbons in Unit:11. The first member of aromatic hydrocarbon is benzene (C6H6) represented by a regular hexagon with a circle inscribed in it.
• Since, all the six hydrogen atom in benzene are equivalent, it can give only one mono-substituted compound (Ex) methyl benzene (C6H5-CH3) which named as toluene.
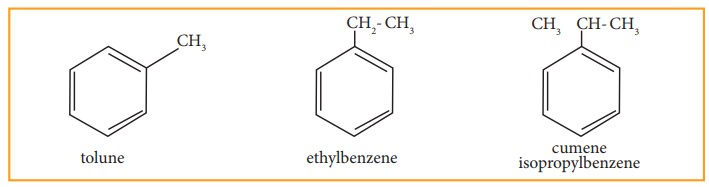
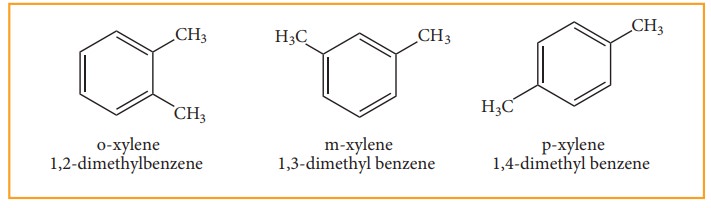
• When di substitution occurs either by a similar monovalent atom or two different atoms or groups in benzene, then three different position isomers are possible. Their relative positions are indicated as ortho (1,2), meta (1,3) and para (1,4). For example, consider dimethyl benzene which is named as xylene.
Related Topics