Chapter: Essential Anesthesia From Science to Practice : Applied physiology and pharmacology : A brief pharmacology related to anesthesia
Nitrous oxide - anesthesia
The gases
Only two anesthetic gases (as opposed to vapors) deserve to be mentioned: nitrous oxide and xenon. Cyclopropane and ethylene are two explosive gases used in the past.
Nitrous oxide
Nitrous oxide has been around for centuries and is still widely used. Yet you will often hear it said that, if nitrous oxide were to be introduced today, it would never pass the FDA’s muster. For this jaundiced view, we can cite several reasons.
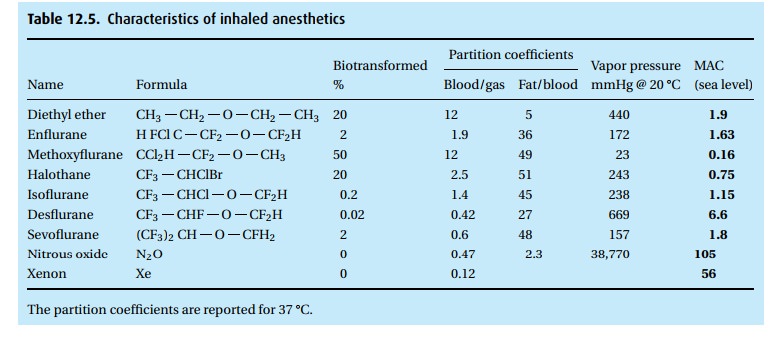
· The gas is a weak anesthetic with a MAC of 105%. Thus, it would require a hyperbaric chamber to administer that concentration with enough oxygen to make it safe. In concentrations up to 70% in oxygen, it is an analgesic rather than a reliable anesthetic.
· Because it is such a weak drug, in the past people tended to give high concen-trations of it, which is another way of saying that it was given with marginal concentrations of oxygen. Modern anesthesia machines will not let you give less than 25% oxygen, but many patients with ventilation/perfusion abnor-malities require a higher FiO2.
· It does some peculiar things to some important enzymes. By oxidizing vitamin-B12-dependent enzymes (methionine and thymidylate synthetase), it inhibits formation of myelin and thymidine (important in DNA synthesis). Prolonged exposure to nitrous oxide has caused neuropathy and megaloblas-tic changes as well as leukopenia. A decreased white count was noticed in tetanus patients requiring prolonged mechanical ventilation during which nitrous oxide was used continuously as an analgesic sedative. Attempting to use this effect to advantage, subsequent experiments with nitrous oxide in leukemic patients confirmed the observation that the gas could reduce the white count. Unfortunately, the effect did not last and upon discontinu-ation of the gas, the cell counts rose back to their pathologic condition. The neuropathic effect of nitrous oxide was observed by a neurologist who saw dentists complaining of different degrees of apraxia, ataxia, and impotence. Exposure to nitrous oxide was the common denominator in these patients. These effects are not observed during the relatively brief use (minutes or hours instead of repeated use or days of exposure) of nitrous oxide in patients undergoing surgical anesthesia.
· Despite its low blood solubility (blood/gas partition coefficient of 0.47), the high concentration of N2O administered (50% to 70% in oxygen) causes many liters to dissolve in the body during a lengthy anesthetic. Because it diffuses readily into air-containing bubbles, nitrous oxide can increase the volume of air in the cuff of an endotracheal tube, the gas in the bowel, a bleb in the lung, or gas in the middle ear. The volume of a closed air space, e.g., pneumothorax, will double in just 10 minutes! The doubling time for bowel is much slower (hours).
· We might also mention that it supports combustion, almost as well as oxygen.
· For neurosurgical procedures, even low-dose and brief exposure to nitrous oxide affects evoked potentials – which we monitor to keep an eye on the integrity of the spinal cord, among other things.
· Based on questionable epidemiologic data and on animal experiments, nitrous oxide has been accused of causing spontaneous abortion in person-nel repeatedly exposed to trace concentrations of the gas. Consequently, maximal acceptable trace concentrations of nitrous oxide in the OR have been established by the government: OSHA calls for a time weighted aver-age concentration of less than 25 parts per million.
· Finally, thrill seekers have extensively abused nitrous oxide, obtaining it legally (and stupidly) in the small whippet cylinders. There, the gas exists in its pure form, that is without oxygen. The ill-informed who inhale it from such a source expose themselves to the double trouble of inhaling a hypoxic gas mixture while breathing a harmful gas.
In fairness, we have to say something positive about the gas. Because of its low solubility, it does not take much time to reach equilibrium between alveolar gas and blood, which translates into fairly rapid induction and emergence with min-imal cardiovascular side effects. Some pediatric dentists like its mild analgesic effect and the fact that it is tasteless and odorless (which is why industry uses it as a propellant for canned whipped cream). In the pediatric dental practice, nitrous oxide is usually administered in concentrations between 30% and 50% in oxygen. Higher concentrations of nitrous oxide given by itself often lead to excite-ment. In anesthetic practice, therefore, we administer the gas together with other CNS depressants, for example thiopental or propofol or a halogenated anesthetic vapor.
Even though it has nothing to do with the pharmacology of nitrous oxide, and everything to do with the fact that we give it in high concentrations (up to 70% – whereas the halogenated agents are given in less than 1/10th that concentration), we need to mention three concepts linked to nitrous oxide: the second gas effect; the augmented inflow effect (also called the concentration effect); and diffusion hypoxia.
The second gas effect
If you administer a high concentration of nitrous oxide to the lungs during induc-tion of anesthesia, much of the gas will go into solution in the body, thereby reducing its partial pressure in the lungs. The sum of all partial pressures will equal barometric pressure. In other words, if a large volume of nitrous oxide van-ishes, any other (second) (anesthetic) gas present in the lung will experience an increase in its partial pressure, which will speed its uptake by the blood.
The augmented inflow or concentration effect
Because of the large uptake of nitrous oxide, the exhaled volume will be dimin-ished, enabling the next breath to have an increased tidal volume to re-establish normal lung volume.
Diffusion hypoxia
After hours of anesthesia with nitrous oxide, many liters of the gas go into solution in the body. At the end of anesthesia, when the patient no longer inhales nitrous oxide, the liters of nitrous oxide in solution will follow their concentration gradient and be delivered to the lung where the gas will displace other gases – including oxygen. Thus, we give oxygen for a few breaths at the end of anesthesia and thus prevent diffusion hypoxia.
Related Topics