Chapter: Biochemistry: The Metabolism of Nitrogen
Nitrogen Metabolism: An Overview
Nitrogen Metabolism: An Overview
We have
seen the structures of many types of compounds that contain nitrogen, including
amino acids, porphyrins, and nucleotides, but we have not discussed their
metabolism. The metabolic pathways we have dealt with up to now have mainly
involved compounds of carbon, hydrogen, and oxygen, such as sugars and fatty
acids. Several important topics can be included in our discussion of the
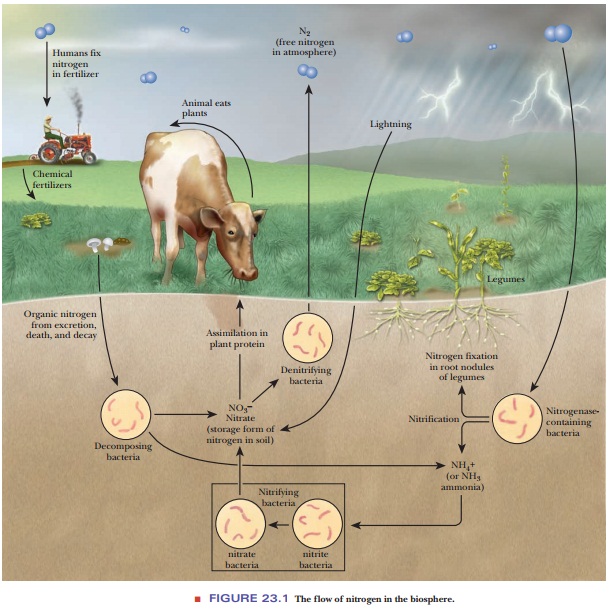
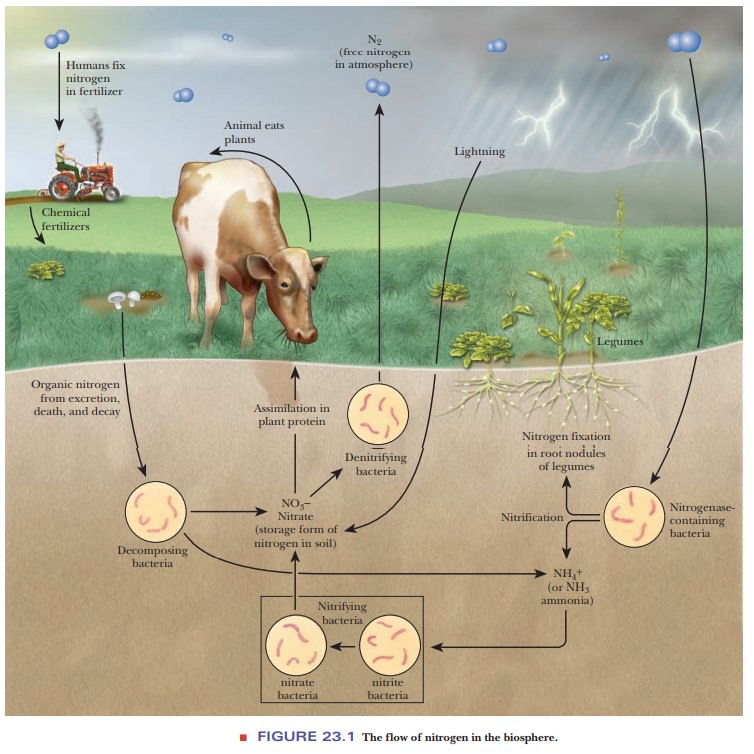
metabolism of nitrogen. The first of these is nitrogen fixation, the process by which inorganic molecular
nitrogen from the atmosphere (N2) is incorporated first into ammonia
and then into organic compounds that are of use to organisms. Nitrate ion (NO3–),
another kind of inorganic nitrogen, is the form in which nitrogen is found in the
soil, and many fertilizers contain nitrates, frequently potassium nitrate. The
process of nitrification (nitrate
reduction to ammonia) provides another way for organisms to obtain nitrogen.
Nitrate ion and nitrite ion (NO2–) are also involved in denitrification reactions, which return
nitrogen to the atmosphere (Figure 23.1).
Ammonia
formed by either pathway, nitrogen fixation or nitrification, enters the
biosphere. Ammonia is converted to organic nitrogen by plants, and organic
nitrogen is passed to animals through food chains. Finally, animal waste
products, such as urea, are excreted and degraded to ammonia by
micro-organisms. The word ammonia
comes from sal ammoniac (ammonium
chlo-ride), which was first prepared from the dung of camels at the temple of
Jupiter Ammon in North Africa. The process of death and decay releases ammonia
in both plants and animals. Denitrifying bacteria reverse the conversion of
ammo-nia to nitrate and then recycle the NO3– as free N2
(Figure 23.1).
The
topic of nitrogen metabolism includes the biosynthesis and breakdown of amino acids, purines, and pyrimidines; also, the metabolism of porphyrins is relatedto that of amino
acids. Many of these pathways, particularly the anabolic ones, are long and
complex. In discussing pathways in which the amount of material is large and
highly detailed, we shall concentrate on the most important points. Specifically,
we shall concentrate on overall patterns and on interesting reac-tions of wide
applicability. We shall also be interested in health-related aspects of this
material. Other reactions will be found at the BiochemistryNow Interactive
website for this text.
Summary
Atmospheric
nitrogen (N2) is not highly reactive, but it must be converted to
ammonia or to nitrates to be biologically useful, first to plants, then to
animals.
Nitrogen
compounds in the biosphere include amino acids, purines, and pyrimidines. Their
pathways for biosynthesis and breakdown tend to be long and complex.
Related Topics