Preparation, Properties, Examples, Uses - Nitric acid | 12th Chemistry : UNIT 3 : p-Block Elements-II
Chapter: 12th Chemistry : UNIT 3 : p-Block Elements-II
Nitric acid
Nitric
acid
Preparation
Nitric acid is prepared
by heating equal amounts of potassium or sodium nitrate with concentrated
sulphuric acid.
KNO3 + H2SO4
→ KHSO4 + HNO3
The temperature is kept
as low as possible to avoid decomposition of nitric acid. The acid condenses to
a fuming liquids which is coloured brown by the presence of a little nitrogen
dioxide which is formed due to the decomposition of nitric acid.
4HNO3 → 4NO2
+ 2H2O + O2
Commercial method of preparation
Nitric acid prepared in
large scales using Ostwald's process. In this method ammonia from Haber’s
process is mixed about 10 times of air. This mixture is preheated and passed
into the catalyst chamber where they come in contact with platinum gauze. The
temperature rises to about 1275 K and the metallic gauze brings about the rapid
catalytic oxidation of ammonia resulting in the formation of NO, which then
oxidised to nitrogen dioxide.
4NH3 + 5O2
→ 4NO + 6H2O + 120 kJ
2NO + O2 → 2NO2
The nitrogen dioxide
produced is passed through a series of adsorption towers. It reacts with water
to give nitric acid. Nitric acid formed is bleached by blowing air.
6NO2 + 3H2O
→ 4HNO3 + 2NO + H2O
Properties
Pure nitric acid is
colourless. It boils at 86 °C. The acid is completely miscible with water
forming a constant boiling mixture (98% HNO3, Boiling point 120.5
°C). Fuming nitric acid contains oxides of nitrogen. It decomposes on exposure
to sunlight or on being heated, into nitrogen dioxide, water and oxygen.
4HNO3 → 4NO2
+ 2H2O + O2
Due to this reaction
pure acid or its concentrated solution becomes yellow on standing.
In most of the
reactions, nitric acid acts as an oxidising agent. Hence the oxidation state
changes from +5 to a lower one. It doesn’t yield hydrogen in its reaction with
metals. Nitric acid can act as an acid, an oxidizing agent and an nitrating
agent.
As an acid: Like other acids it
reacts with bases and basic oxides to form salts and water
ZnO + 2HNO3 →
Zn(NO3 )2 + H2O
3FeO + 10HNO3
→ 3Fe(NO3 )3 + NO + 5 H2O
As an oxidising agent: The nonmetals like
carbon, sulphur, phosphorus and iodine are oxidised by nitric acid.
C + 4HNO3 → 2H2O
+ 4NO2 + CO2
S + 2HNO3 → H2SO4
+ 2NO
P4 + 20HNO3
→ 4H3PO4 + 4H2O + 20NO2
3I2 + 10HNO3
→ 6HIO3 + 10NO + 2H2O
HNO3 + F2
→ HF + NO3F
3H2S + 2HNO3
→ 3S + 2NO + 4H2O
As an nitrating agent: In organic compounds
replacement of a –H atom with –NO2 is often referred
as nitration. For example.
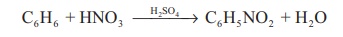
C6H6
+ HNO3 → H 2 SO4 →
C6H5 NO2 + H2O
Nitration takes place
due to the formation of nitronium ion
HNO3 + H2SO4
→ NO2+
+ H3O+ + HSO4−
Action of nitric acid on metals
All metals with the
exception of gold, platinum, rhodium, iridium and tantalum reacts with nitric
acid. Nitric acid oxidises the metals. Some metals such as aluminium, iron,
cobalt, nickel and chromium are rendered passive in concentrated acid due to
the formation of a layer of their oxides on the metal surface, which prevents
the nitric acid from reacting with pure metal.
With weak
electropositive metals like tin, arsenic, antimony, tungsten and molybdenum,
nitric acid gives metal oxides in which the metal is in the higher oxidation
state and the acid is reduced to a lower oxidation state. The most common
products evolved when nitric acid reacts with a metal are gases NO2,
NO and H2O. Occasionally N2, NH2OH and NH3
are also formed.
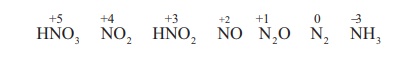
The reactions of metals
with nitric acid are explained in 3 steps as follows:
Primary reaction: Metal nitrate is formed
with the release of nascent hydrogen
M + HNO3 → MNO3
+ (H)
Secondary reaction: Nascent hydrogen
produces the reduction products of nitric acid.
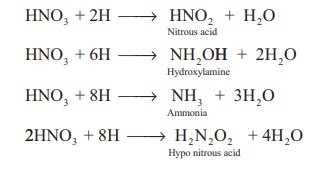
Tertiary reaction: The secondary products
either decompose or react to give final products
Decomposition of the secondary:
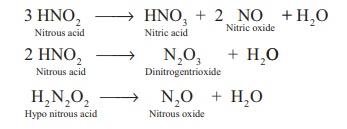
Reaction of secondary products:
HNO2 + NH3 → N2 + 2H2O
HNO2 + NH2OH → N2O + 2H2O
HNO2 + HNO3 → 2NO2 + H2O
Examples:
Copper reacts with
nitric acid in the following manner
3Cu + 6HNO3 →
3Cu(NO3 )2 + 6(H)
6(H) + 3HNO3 →
3HNO2 + 3H2O
3HNO2 → HNO3
+ 2NO + H2O
overall reation
3Cu + 8HNO3 →
3Cu(NO3 )2 + 2NO + 4H2O
The concentrated acid
has a tendency to form nitrogen dioxide
Cu + 4HNO3 → Cu(NO3
)2 + 2NO2 + 2H2O
Magnesium reacts with
nitric acid in the following way
4Mg + 8HNO3 →
4Mg(NO3 )2 + 8[H]
HNO3 + 8H → NH3
+ 3H2O
HNO3 + NH3
→ NH4 NO3
overall reaction
4Mg + 10HNO3 →
4Mg(NO3 )2 + NH4 NO3 + 3H2O
If the acid is diluted
we get N2O
4Mg + 10HNO3 →
4Mg(NO3 )2 + N2O + 5H2O
Uses of nitric acid:
·
Nitric acid is used as a oxidising agent and in the preparation of
aquaregia.
·
Salts of nitric acid are used in photography (AgNO3)
and gunpowder for firearms. (NaNO3)
Related Topics