Chapter: Basic & Clinical Pharmacology : Vasodilators & the Treatment of Angina Pectoris
Nitrates & Nitrites
NITRATES & NITRITES
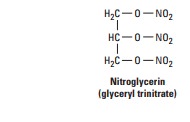
Chemistry
These
agents are simple nitric and nitrous acid esters of polyalcohols. Nitroglycerin may be considered the
prototype of the group. Although nitroglycerin is used in the manufacture of
dynamite, the systemic formulations used in medicine are not explosive. The
conventional sublingual tablet form of nitroglycerin may lose potency when
stored as a result of volatilization and adsorption to plastic surfaces.
Therefore, it should be kept in tightly closed glass containers. Nitroglycerin
is not sensitive to light.
All
therapeutically active agents in the nitrate group appear to have identical
mechanisms of action and similar toxicities, although susceptibility to
tolerance may vary. Therefore, pharma-cokinetic factors govern the choice of
agent and mode of therapy when using the nitrates.
Pharmacokinetics
The
liver contains a high-capacity organic nitrate reductase that removes nitrate
groups in a stepwise fashion from the parentmolecule and ultimately inactivates
the drug. Therefore, oral bio-availability of the traditional organic nitrates
(eg, nitroglycerin and isosorbide
dinitrate) is low (typically<10–20%). For this rea-son, the sublingual route, which avoids
the first-pass effect, is preferred for achieving a therapeutic blood level
rapidly. Nitroglycerin and isosorbide dinitrate both are absorbed effi-ciently
by this route and reach therapeutic blood levels within a few minutes. However,
the total dose administered by this route must be limited to avoid excessive
effect; therefore, the total dura-tion of effect is brief (15–30 minutes). When
much longer dura-tion of action is needed, oral preparations can be given that
contain an amount of drug sufficient to result in sustained sys-temic blood
levels of the parent drug plus active metabolites. Other routes of
administration available for nitroglycerin include transdermal and buccal
absorption from slow-release preparations (described below). Amyl nitrite and related nitrites are
highly volatile liquids.Amyl nitrite is available in fragile glass ampules
packaged in a protective cloth covering. The ampule can be crushed with the
fingers, resulting in rapid release of vapors inhalable through the cloth
covering. The inhalation route provides very rapid absorp-tion and, like the
sublingual route, avoids the hepatic first-pass effect. Because of its unpleasant
odor and short duration of action, amyl nitrite is now obsolete for angina.
Once
absorbed, the unchanged nitrate compounds have half-lives of only 2–8 minutes.
The partially denitrated metabolites have much longer half-lives (up to 3
hours). Of the nitroglycerin metabolites (two dinitroglycerins and two
mononitro forms), the 1,2-dinitro derivative has significant vasodilator
efficacy and prob-ably provides most of the therapeutic effect of orally
administered nitroglycerin. The 5-mononitrate metabolite of isosorbide
dinitrate is an active metabolite of the latter drug and is available for oral
use as isosorbide mononitrate. It
has a bioavailability of 100%.Excretion, primarily in the form of glucuronide
derivatives of the denitrated metabolites, is largely by way of the kidney.
Pharmacodynamics
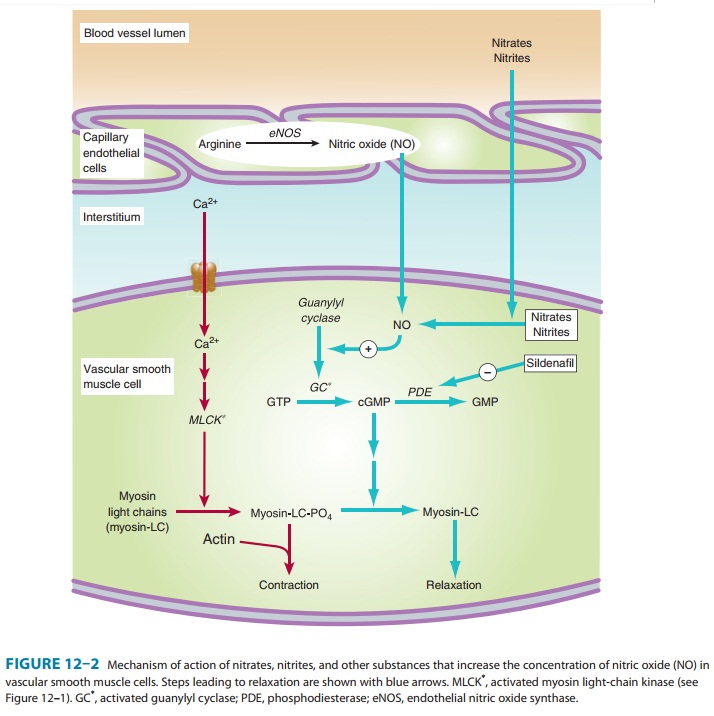
A. Mechanism of Action in Smooth Muscle
After
more than a century of study, the mechanism of action of nitroglycerin is still
not fully understood. There is general agree-ment that the drug must be
bioactivated with the release of nitric oxide. Unlike nitroprusside and some
other direct nitric oxide donors, nitroglycerin activation requires enzymatic
action. Nitroglycerin can be denitrated by glutathione S-transferase in smooth muscle and other cells. A mitochondrial
enzyme, aldehyde dehydrogenase isoform 2 (ALDH2) and possibly isoform 3, ALDH3,
is also capable of activating nitroglycerin and releasing nitric oxide. The
differential selectivity of glutathione S-transferase
and ALDH2 for different organic nitrates suggests that the ALDH2 may be the
more important enzyme for nitroglycerin bioactivation. Free nitrite ion is
released, which is then converted to nitric
oxide . Nitric oxide (probably com-plexed with cysteine) combines with the
heme group of soluble guanylyl cyclase, activating that enzyme and causing an
increase in cGMP. As shown in Figure 12–2, formation of cGMP represents a first
step toward smooth muscle relaxation. The production of prostaglandin E or
prostacyclin (PGI2) and membrane hyperpo-larization may also be
involved. There is no evidence that auto-nomic receptors are involved in the
primary nitrate response. However, autonomic reflex responses, evoked when hypotensive doses are given, are
common.
As
described in the following text, tolerance is an important consideration in the
use of nitrates. Although tolerance may be caused in part by a decrease in
tissue sulfhydryl groups, eg, on cysteine, it can be only partially prevented
or reversed with a sulfhydryl-regenerating agent. Increased generation of
oxygen free radicals during nitrate therapy may be another important mecha-nism
of tolerance. Recent evidence suggests that diminished avail-ability of
calcitonin gene-related peptide (CGRP, a potent vasodilator) is also associated
with nitrate tolerance. Nicorandil and several other investigational
antianginal agents appear to combine the activity of nitric oxide release with
potas-sium channel-opening action, thus providing an additional mechanism for
causing vasodilation. Nitroglycerin has not been reported to open potassium
channels.
B. Organ System Effects
Nitroglycerin
relaxes all types of smooth muscle regardless of the cause of the preexisting
muscle tone (Figure 12–3). It has practi-cally no direct effect on cardiac or
skeletal muscle.
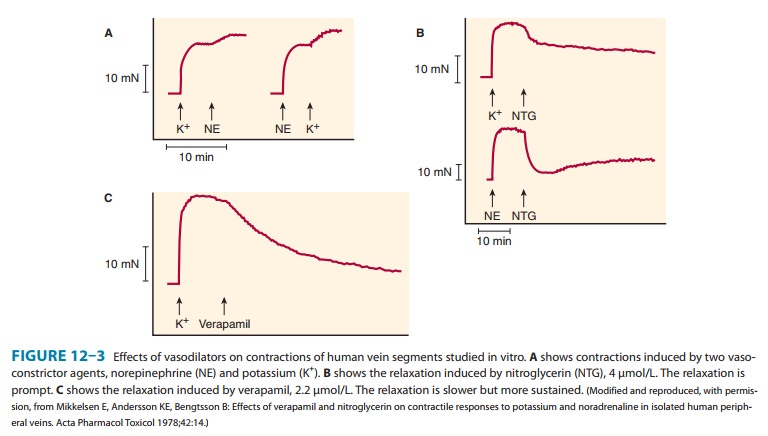
Vascular smooth muscle— All segments of the
vascularsystem from large arteries through large veins relax in response to
nitroglycerin. Most evidence suggests a gradient of response, with veins
responding at the lowest concentrations, arteries at slightly higher ones. The
epicardial coronary arteries are sensitive, but concentric atheromas can
prevent significant dilation. On the other hand, eccentric lesions permit an
increase in flow when nitrates relax the smooth muscle on the side away from
the lesion. Arterioles and precapillary sphincters are dilated least, partly
because of reflex responses and partly because different vessels vary in their
ability to release nitric oxide from the drug.
A
primary direct result of an effective dose of nitroglycerin is marked relaxation
of veins with increased venous capacitance and decreased ventricular preload.
Pulmonary vascular pressures and heart size are significantly reduced. In the
absence of heart failure, cardiac output is reduced. Because venous capacitance
is increased, orthostatic hypotension may be marked and syncope can result.
Dilation of large epicardial coronary arteries may improve oxygen delivery in
the presence of eccentric atheromas. Temporal artery pulsations and a throbbing
headache associated with meningeal artery pulsations are common effects of
nitroglycerin and amyl nitrite. In heart failure, preload is often abnormally
high; the nitrates and other vasodilators, by reducing preload, may have a
beneficial effect on cardiac output in this condition .
The
indirect effects of nitroglycerin consist of those compensa-tory responses
evoked by baroreceptors and hormonal mecha-nisms responding to decreased
arterial pressure (see Figure 6–7); this often results in tachycardia and
increased cardiac contractility. Retention of salt and water may also be
significant, especially with intermediate- and long-acting nitrates. These
compensatory responses contribute to the development of tolerance.
In
normal subjects without coronary disease, nitroglycerin can induce a significant,
if transient, increase in total coronary blood flow. In contrast, there is no
evidence that total coronary flow is increased in patients with angina due to
atherosclerotic obstruc-tive coronary artery disease. However, some studies
suggest that redistribution of
coronary flow from normal to ischemic regionsmay play a role in nitroglycerin’s
therapeutic effect. Nitroglycerin also exerts a weak negative inotropic effect
on the heart via nitric oxide.
Other smooth muscle organs—Relaxation of
smoothmuscle of the bronchi, gastrointestinal tract (including biliary system),
and genitourinary tract has been demonstrated experi-mentally. Because of their
brief duration, these actions of the nitrates are rarely of any clinical value.
During recent decades, the use of amyl nitrite and isobutyl nitrite (not
nitrates) by inhalation as recreational (sex-enhancing) drugs has become
popular with some segments of the population. Nitrites readily release nitric
oxide in erectile tissue as well as vascular smooth muscle and activate
guanylyl cyclase. The resulting increase in cGMP causes dephospho-rylation of
myosin light chains and relaxation (Figure 12–2), which enhances erection. The
pharmacologic approach to erectile dys-function is are discussed in the Box:
Drugs Used in the Treatment of Erectile Dysfunction.
Action on platelets—Nitric oxide released
from nitroglycerinstimulates guanylyl cyclase in platelets as in smooth muscle.
The increase in cGMP that results is responsible for a decrease in platelet
aggregation. Unfortunately, recent prospective trials have estab-lished no
survival benefit when nitroglycerin is used in acute myo-cardial infarction. In
contrast, intravenous nitroglycerin may be of value in unstable angina, in part
through its action on platelets.
Other effects—Nitriteion reacts with hemoglobin (whichcontains ferrous iron) to
produce methemoglobin (which contains ferric iron). Because methemoglobin has a
very low affinity for oxygen, large doses of nitrites can result in
pseudocyanosis, tissue hypoxia, and death. Fortunately, the plasma level of
nitrite result-ing from even large doses of organic and inorganic nitrates is
too low to cause significant methemoglobinemia in adults. In nursing infants,
the intestinal flora is capable of converting significant amounts of inorganic
nitrate, eg, from well water, to nitrite ion. In addition, sodium nitrite is
used as a curing agent for meats, eg,
corned
beef. Thus, inadvertent exposure to large amounts of nitrite ion can occur and
may produce serious toxicity.
One therapeutic application of this otherwise toxic effect of nitrite has been discovered. Cyanide poisoning results from com-plexing of cytochrome iron by the CN− ion. Methemoglobin iron has a very high affinity for CN−; thus, administration of sodium nitrite (NaNO2) soon after cyanide exposure regenerates active cytochrome. The cyanmethemoglobin produced can be further detoxified by the intravenous administration of sodium thiosulfate (Na2S2O3); this results in formation of thiocyanate ion (SCN−), a less toxic ion that is readily excreted. Methemoglobinemia, if excessive, can be treated by giving methylene blue intravenously. This antidotal procedure is now being replaced by hydroxocobala-min, a form of vitamin B12, which also has a very high affinity for cyanide and converts it to another form of vitamin B12.
Toxicity & Tolerance
A. Acute Adverse Effects
The
major acute toxicities of organic nitrates are direct extensions of therapeutic
vasodilation: orthostatic hypotension, tachycardia, and throbbing headache.
Glaucoma, once thought to be a con-traindication, does not worsen, and nitrates
can be used safely in the presence of increased intraocular pressure. Nitrates
are con-traindicated, however, if intracranial pressure is elevated. Rarely,
transdermal nitroglycerin patches have ignited when external defi-brillator
electroshock was applied to the chest of patients in ven-tricular fibrillation.
Such patches should be removed before use of external defibrillators to prevent
superficial burns.
B. Tolerance
With
continuous exposure to nitrates, isolated smooth muscle may develop complete
tolerance (tachyphylaxis), and the intact human becomes progressively more
tolerant when long-acting preparations (oral, transdermal) or continuous
intravenous infu-sions are used for more than a few hours without interruption.
The mechanisms by which tolerance develops are not completely understood. As
previously noted, diminished release of nitric oxide resulting from reduced
bioactivation may be partly respon-sible for tolerance to nitroglycerin.
Systemic compensation also plays a role in tolerance in the intact human.
Initially, significant sympathetic discharge occurs, and after one or more days
of therapy with long-acting nitrates, retention of salt and water may reverse
the favorable hemodynamic changes normally caused by nitroglycerin. Tolerance
does not occur equally with all nitric oxide donors. Nitroprusside, for
example, retains activity over long periods. Other organic nitrates appear to
be less susceptible than nitroglyc-erin to the development of tolerance. In
cell-free systems, soluble guanylate cyclase is inhibited, possibly by
nitrosylation of the enzyme, only after prolonged exposure to exceedingly high
nitro-glycerin concentrations. In contrast, treatment with antioxidants that
protect ALDH2 and similar enzymes appears to prevent or reduce tolerance. This
suggests that tolerance is a function of diminished bioactivation of organic
nitrates and to a lesser degree, a loss of soluble guanylate cyclase
responsiveness to nitric oxide.
Continuous
exposure to high levels of nitrates can occur in the chemical industry,
especially where explosives are manufactured. When contamination of the
workplace with volatile organic nitrate compounds is severe, workers find that
upon starting their work week (Monday), they suffer headache and transient
dizziness (“Monday disease”). After a day or so, these symptoms disappear owing
to the development of tolerance. Over the weekend, when exposure to the
chemicals is reduced, tolerance disappears, so symp-toms recur each Monday.
Other hazards of industrial exposure, including dependence, have been reported.
There is no evidence that physical dependence develops as a result of the therapeutic use of short-acting nitrates
for angina, even in large doses.
C. Carcinogenicity of Nitrate and Nitrite Derivatives
Nitrosamines
are small molecules with the structure R2–N–NO formed from the
combination of nitrates and nitrites with amines. Some nitrosamines are
powerful carcinogens in animals, appar-ently through conversion to reactive
derivatives. Although there is no direct proof that these agents cause cancer
in humans, there is a strong epidemiologic correlation between the incidence of
esophageal and gastric carcinoma and the nitrate content of food in certain
cultures. Nitrosamines are also found in tobacco and in cigarette smoke. There
is no evidence that the small doses of nitrates used in the treatment of angina
result in significant body levels of nitrosamines.
Mechanisms of Clinical Effect
The
beneficial and deleterious effects of nitrate-induced vasodila-tion are
summarized in Table 12–2.
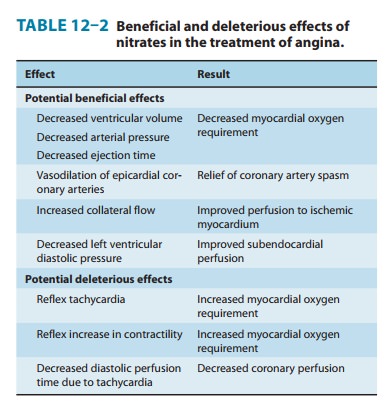
A. Nitrate Effects in Angina of Effort
Decreased
venous return to the heart and the resulting reduction of intracardiac volume
are important beneficial hemodynamic effects of nitrates. Arterial pressure
also decreases. Decreased intraventricular pressure and left ventricular volume
are associ-ated with decreased wall tension (Laplace relation) and decreased
myocardial oxygen requirement. In rare instances, a paradoxical increase in myocardial oxygen demand may
occur as a result ofexcessive reflex tachycardia and increased contractility.
Intracoronary,
intravenous, or sublingual nitrate administra-tion consistently increases the
caliber of the large epicardial coro-nary arteries except where blocked by
concentric atheromas. Coronary arteriolar resistance tends to decrease, though
to a lesser extent. However, nitrates administered by the usual systemic routes
may decrease overall coronary blood
flow (and myocardial oxygen consumption) if cardiac output is reduced due to
decreased venous return. The reduction in oxygen consumption is the major
mechanism for the relief of effort angina.
B. Nitrate Effects in Variant Angina
Nitrates
benefit patients with variant angina by relaxing the smooth muscle of the
epicardial coronary arteries and relieving coronary artery spasm.
C. Nitrate Effects in Unstable Angina
Nitrates
are also useful in the treatment of the acute coronary syndrome of unstable
angina, but the precise mechanism for their beneficial effects is not clear.
Because both increased coronary vascular tone and increased myocardial oxygen
demand can pre-cipitate rest angina in these patients, nitrates may exert their
beneficial effects both by dilating the epicardial coronary arteries and by
simultaneously reducing myocardial oxygen demand. As previously noted,
nitroglycerin also decreases platelet aggregation, and this effect may be of
importance in unstable angina.
Clinical Use of Nitrates
Some
of the forms of nitroglycerin and its congeners are listed in Table 12–3.
Because of its rapid onset of action (1–3 minutes), sublingual nitroglycerin is
the most frequently used agent for the immediate treatment of angina. Because
its duration of action is short (not exceeding 20–30 minutes), it is not
suitable for main-tenance therapy. The onset of action of intravenous
nitroglycerin is also rapid (minutes), but its hemodynamic effects are quickly
reversed when the infusion is stopped. Clinical use of intravenous
nitroglycerin is therefore restricted to the treatment of severe, recurrent
rest angina. Slowly absorbed preparations of nitroglyc-erin include a buccal
form, oral preparations, and several transder-mal forms. These formulations
have been shown to provide blood concentrations for long periods but, as noted
above, this leads to the development of tolerance.
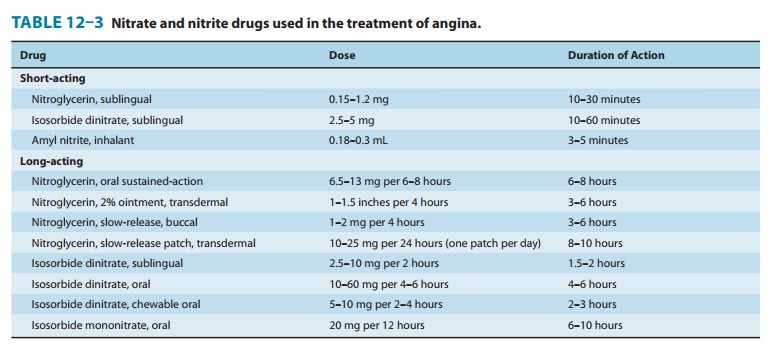
The hemodynamic effects of sublingual or chewable isosorbide dinitrate and the oral organic nitrates are similar to those of nitro-glycerin given by the same route. The recommended dosage sched-ules for commonly used long-acting nitrate preparations, along with their durations of action, are listed in Table 12–3. Although transdermal administration may provide blood levels of nitroglyc-erin for 24 hours or longer, the full hemodynamic effects usually do not persist for more than 6–8 hours. The clinical efficacy of slow-release forms of nitroglycerin in maintenance therapy of angina is thus limited by the development of significant tolerance. Therefore, a nitrate-free period of at least 8 hours between doses should be observed to reduce or prevent tolerance.
OTHER NITRO-VASODILATORS
Nicorandil is a nicotinamide nitrate ester that has
vasodilatingproperties in normal coronary arteries but more complex effects in
patients with angina. Clinical studies suggest that it reduces both preload and
afterload. It also provides some myocardial protection via preconditioning by
activation of cardiac KATP channels. One large trial showed a
significant reduction in relative risk of fatal and nonfatal coronary events in
patients receiving the drug. Nicorandil is currently approved for use in the
treatment of angina in Europe and Japan and has been submitted for approval in
the USA.
Related Topics