Chapter: Introduction to Human Nutrition: The Vitamins
Niacin - Human Nutrition
Niacin
Niacin is not strictly a vitamin, since it can be synthesized in the body from the essential amino acid tryptophan. Indeed, it is only when tryptophan metabo-lism is deranged that dietary preformed niacin becomes important. Nevertheless, niacin was discov-ered as a nutrient during studies of the deficiency disease pellagra, which was a major public health problem in the southern USA throughout the first half of the twentieth century, and continued to be a problem in parts of India and sub-Saharan Africa until the 1990s.
Vitamers and niacin equivalents
Two compounds, nicotinic acid and nicotinamide, have the biological activity of niacin. When nicotinic acid was discovered as the curative and preventive factor for pellagra, it was already known as a chemical compound, and was therefore never assigned a number among the B vitamins. The name niacin was coined in the USA when it was decided to enrich maize meal with the vitamin to prevent pellagra; it was considered that the name nicotinic acid was not desirable because of its similarity to nicotine. In the USA the term niacin is commonly used to mean spe-cifically nicotinic acid, and nicotinamide is known as niacinamide; elsewhere “niacin” is used as a generic descriptor for both vitamers. Figure 8.10 shows the structures of nicotinic acid and niacin, as well as the nicotinamide nucleotide coenzymes, NAD and NADP.
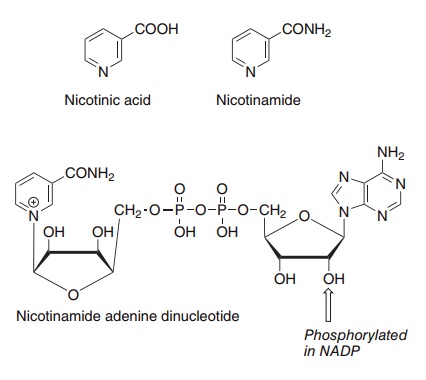
Figure 8.10 The niacin vitamers, nicotinic acid and nicotinamide, and the coenzyme nicotinamide adenine dinucleotide.
The nicotinamide ring of NAD can be synthesized in the body from the essential amino acid tryptophan. In adults almost all of the dietary intake of trypto-phan, apart from the small amount that is used for net new protein synthesis, and synthesis of the neu-rotransmitter serotonin, is metabolized by this pathway, and hence is potentially available for NAD synthesis.
Several studies have investigated the equivalence of dietary tryptophan and preformed niacin as precur-sors of the nicotinamide nucleotides, generally by determining the excretion of niacin metabolites in response to test doses of the precursors, in subjects maintained on deficient diets. The most extensive such study was that of Horwitt et al. in 1956. They found that there was a considerable variation between subjects in the response to tryptophan and niacin, and in order to allow for this individual variation they proposed the ratio of 60 mg of tryptophan equivalent to 1 mg of preformed niacin. Changes in hormonal status may result in considerable changes in this ratio, with between 7 and 30 mg of dietary tryptophan being equivalent to 1 mg of preformed niacin in late pregnancy.
The niacin content of foods is generally expressed as mg niacin equivalents; 1 mg niacin equivalent = mg preformed niacin + 1/60 × mg tryptophan. Because most of the niacin in cereals is biologically unavailable , it is conventional to ignore pre-formed niacin in cereal products.
Because endogenous synthesis from tryptophan is more important than preformed dietary niacin, the main dietary sources of niacin are generally those that are also rich sources of protein. It is only when the dietary staple is a cereal such as maize, which is remarkably lacking in tryptophan, that problems of deficiency occur. Trigonelline in coffee beans is demethylated to nicotinic acid during roasting, and moderate coffee consumption may meet a significant proportion of niacin requirements.
Unavailable niacin in cereals
Chemical analysis reveals niacin in cereals (largely in the bran), but this is biologically unavailable, since it is bound as niacytin – nicotinoyl esters to a variety of macromolecules. In wheat bran some 60% is esteri-fied to polysaccharides, and the remainder to poly-peptides and glycopeptides.
Treatment of cereals with alkali (e.g., by soaking overnight in calcium hydroxide solution, as is the tra-ditional method for the preparation of tortillas in Mexico) and baking with alkaline baking powder releases much of the nicotinic acid. This may explain why pellagra has always been rare in Mexico, despite the fact that maize is the dietary staple.
Up to 10% of the niacin in niacytin may be biologi-cally available as a result of hydrolysis by gastric acid.
Absorption and metabolism
Niacin is present in tissues, and therefore in foods, largely as the nicotinamide nucleotides. The post-mortem hydrolysis of NAD(P) is extremely rapid in animal tissues, so it is likely that much of the niacin of meat (a major dietary source of the preformed vitamin) is free nicotinamide.
Nicotinamide nucleotides present in the intestinal lumen are not absorbed as such, but are hydrolyzed to free nicotinamide. Many intestinal bacteria have high nicotinamide deamidase activity, and a significant proportion of dietary nicotinamide may be deamidated in the intestinal lumen. Both nicotinic acid and nicotinamide are absorbed from the small intestine by a sodium-dependent saturable process.
The nicotinamide nucleotide coenzymes can be synthesized from either of the niacin vitamers and from quinolinic acid, an intermediate in the metabo-lism of tryptophan. In the liver, synthesis of the coen-zymes increases with increasing intake of tryptophan, but not preformed niacin. The liver exports nicotin-amide, derived from turnover of coenzymes, for uptake by other tissues.
Catabolism of NAD(P)
●NAD glycohydrolase, which releases nicotinamide and ADP-ribose;
●NAD pyrophosphatase, which releases nicotin-amide mononucleotide; this can be either hydro-lyzed by NAD glycohydrolase to release nicotin-amide, or reutilized to form NAD;
●ADP-ribosyltransferases;
●poly(ADP-ribose) polymerase.
The activation of ADP-ribosyltransferase and poly(ADP-ribose) polymerase by toxins, oxidative stress or DNA damage may result in considerable depletion of intracellular NAD(P), and may indeed provide a protective mechanism to ensure that cells that have suffered very severe DNA damage die as a result of NAD(P) depletion. The administration of DNA-breaking carcinogens to experimental animals results in the excretion of large amounts of nicotin-amide metabolites and depletion of tissue NAD(P); addition of the compounds to cells in culture has a similar effect. Chronic exposure to such carcinogens and mycotoxins may be a contributory factor in the etiology of pellagra when dietary intakes of trypto-phan and niacin are marginal.
Urinary excretion of niacin and metabolites
Under normal conditions there is little or no urinary excretion of either nicotinamide or nicotinic acid.
This is because both vitamers are actively reabsorbed from the glomerular filtrate. It is only when the con-centration is so high that the reabsorption mecha-nism is saturated that there is any significant excre-tion of niacin.
Nicotinamide in excess of requirements for NAD synthesis is methylated by nicotinamide N-methyltransferase. N1-Methylnicotinamide is actively secreted into the urine by the proximal renal tubules. N1-Methylnicotinamide can also be meta-bolized further, to yield methylpyridone-2- and 4-carboxamides.
Nicotinamide can also undergo oxidation to nico-tinamide N-oxide when large amounts are ingested. Nicotinic acid can be conjugated with glycine to form nicotinuric acid (nicotinoyl-glycine) or may be meth-ylated to trigonelline (N1-methylnicotinic acid). It is not clear to what extent urinary excretion of trigonel-line reflects endogenous methylation of nicotinic acid, since there is a significant amount of trigonelline in foods, which may be absorbed, but cannot be utilized as a source of niacin, and is excreted unchanged.
Metabolic functions of niacin
The best-defined role of niacin is in the metabolism of metabolic fuels, as the functional nicotinamide part of the coenzymes NAD and NADP, which play a major role in oxidation and reduction reactions. The oxi-dized coenzymes have a positive charge on the nico-tinamide ring nitrogen and undergo a two-electron reduction. The oxidized forms are conventionally shown as NAD(P)+ and the reduced forms either as NAD(P)H2 or, more correctly, as NAD(P)H + H+, since although it is a two-electron reduction, only one proton is incorporated into the ring, the other remain-ing associated with the coenzyme.
In general, NAD+ acts as an electron acceptor in energy-yielding metabolism, being oxidized by the mitochondrial electron transport chain, while the major coenzyme for reductive synthetic reactions is NADPH. An exception to this general rule is the pentose phosphate pathway of glucose metabolism, which results in the reduction of NADP+ to NADPH, and is the source of half the reductant for fatty acid synthesis.
In addition to its coenzyme role, NAD is the source of ADP-ribose for the ADP-ribosylation of a variety of proteins and poly(ADP-ribosylation) and hence activation of nucleoproteins involved in the DNA repair mechanism.
In the nucleus, poly(ADP-ribose)polymerase is activated by binding to breakage points in DNA. The enzyme is involved in activation of the DNA repair mechanism in response to strand breakage caused by radical attack or UV radiation. In cells that have suf-fered considerable DNA damage, the activation of poly (ADP-ribose) polymerase may deplete intracel-lular NAD to such an extent that ATP formation is impaired, leading to cell death.
ADP-ribose cyclase catalyzes the formation of cyclic ADP-ribose from NAD, and of nicotinic acid adenine dinucleotide phosphate from NADP (by cat-alyzing the exchange of nicotinamide for nicotinic acid). Both of these compounds act to raise cytosolic calcium concentrations by releasing calcium from intracellular stores, acting as second messengers in response to nitric oxide, acetylcholine, and other neurotransmitters.
Pellagra: a disease of tryptophan and niacin deficiency
Pellagra became common in Europe when maize was introduced from the New World as a convenient high-yielding dietary staple, and by the late nineteenth century it was widespread throughout southern Europe, north and south Africa, and the southern USA. The proteins of maize are particularly lacking in tryptophan, and as with other cereals little or none of the preformed niacin is biologically available.
Pellagra is characterized by a photosensitive der-matitis, like severe sunburn, typically with a butterfly-like pattern of distribution over the face, affecting all parts of the skin that are exposed to sunlight. Similar skin lesions may also occur in areas not exposed to sunlight, but subject to pressure, such as the knees, elbows, wrists, and ankles. Advanced pellagra is also accompanied by dementia (more correctly a depres-sive psychosis), and there may be diarrhea. Untreated pellagra is fatal.
The depressive psychosis is superficially similar to schizophrenia and the organic psychoses, but clini-cally distinguishable by sudden lucid phases that alternate with the most florid psychiatric signs. It is probable that these mental symptoms can be explained by a relative deficit of the essential amino acid tryp-tophan, and hence reduced synthesis of the neuro-transmitter 5-hydroxytryptamine (serotonin), and not to a deficiency of niacin per se.
Additional factors in the etiology of pellagra
Pellagra also occurs in India among people whose dietary staple is jowar (Sorghum vulgare), even though the protein in this cereal contains enough tryptophan to permit adequate synthesis of NAD. Here the problem seems to be the relative excess of leucine in the protein, which can inhibit the synthesis of NAD from tryptophan. It is likely that leucine is a factor in the etiology of pellagra only when the dietary intakes of both tryptophan and niacin are low, a condition that may occur when sorghum is the dietary staple, especially at times of food shortage.
Although the nutritional etiology of pellagra is well established, and tryptophan or niacin will prevent or cure the disease, additional factors, including defi-ciency of riboflavin or vitamin B6, both of which are required for synthesis of NAD from tryptophan, may be important when intakes of tryptophan and niacin are only marginally adequate.
During the first half of the twentieth century, of the 87 000 people who died from pellagra in the USA there were twice as many women as men. Reports of individual outbreaks of pellagra, both in the USA and more recently elsewhere, show a similar gender ratio. This may well be the result of inhibition of trypto-phan metabolism by estrogen metabolites, and hence reduced synthesis of NAD from tryptophan.
Several bacterial, fungal and environmental toxins activate ADP-ribosyltransferase or poly(ADP-ribose) polymerase, and it is possible that chronic exposure to such toxins will deplete tissue NAD(P) and hence be a contributory factor in the development of pella-gra when intakes of tryptophan and niacin are marginal.
Niacin requirements
On the basis of depletion/repletion studies in which the urinary excretion of niacin metabolites was mea-sured after feeding tryptophan or preformed niacin, the average requirement for niacin is 1.3 mg of niacin equivalents/MJ energy expenditure, and reference intakes are based on 1.6 mg/MJ.
Average intakes of tryptophan in Western diets will more than meet requirements without the need for a dietary source of preformed niacin.
Assessment of niacin status
Although the nicotinamide nucleotide coenzymes function in a large number of oxidation and reduc-tion reactions, this cannot be exploited as a means of assessing the state of the body’s niacin reserves, because the coenzymes are not firmly attached to their apoenzymes, as are thiamin pyrophosphate, riboflavin, and pyridoxal phosphate, but act as cosub-strates of the reactions, binding to and leaving the enzyme as the reaction proceeds. No specific meta-bolic lesions associated with NAD(P) depletion have been identified.
The two methods of assessing niacin nutritional status are measurement of the ratio of NAD/ NADP in red blood cells and the urinary excretion of niacin metabolites, neither of which is wholly satisfactory.
Niacin toxicity
Nicotinic acid has been used to lower blood triacyl-glycerol and cholesterol in patients with hyperlipid-emia. However, relatively large amounts are required (of the order of 1–6 g/day, compared with reference intakes of 18–20 mg/day). At this level of intake, nicotinic acid causes dilatation of blood vessels and flushing, with skin irritation, itching, and a burning sensation. This effect wears off after a few days.
High intakes of both nicotinic acid and nicotin-amide, in excess of 500 mg/day, also cause liver damage, and prolonged use can result in liver failure. This is especially a problem with sustained-release preparations of niacin, which permit a high blood level to be maintained for a relatively long time.
Related Topics