Chapter: Essential Anesthesia From Science to Practice : Clinical management : Regional anesthesia
Neuraxial anesthesia
Neuraxial anesthesia
Neuraxial
anesthesia involves the placement of local anesthetics and/or opioids into the
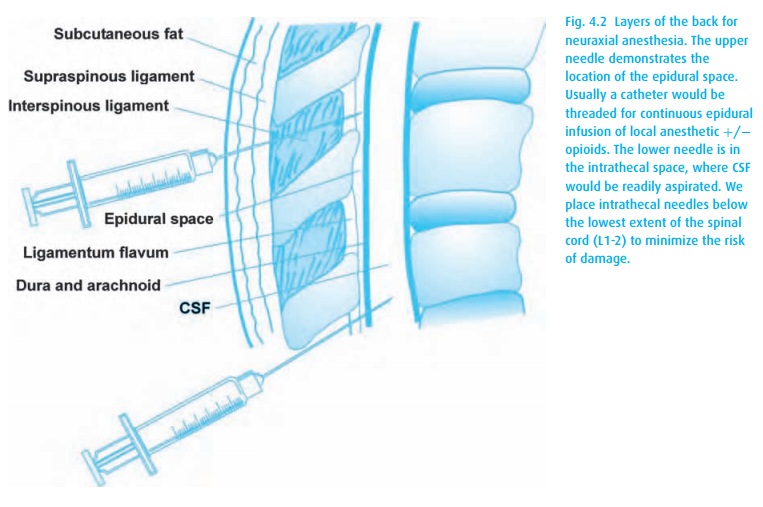
intrathecal (subarachnoid) or epidural space (Fig. 4.2),
either by a single injection or by a continuous infusion catheter technique.
The medications act directly on the spinal cord and, for epidurals, also on the
spinal roots. This results in decreased transmission of impulses through the
various nerves (Table 4.1).
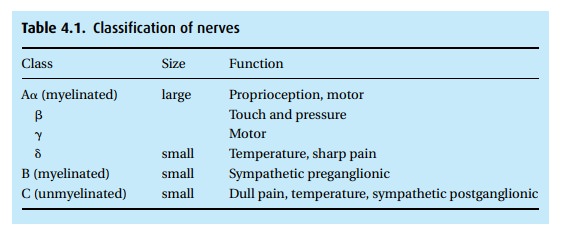
Some
local anesthetics have differential effects on various nerve types. For most
applications, we would prefer to block only the pain impulses, but no agent is
quite that specific. Bupivacaine blocks sensory more than motor fibers and is
the agent of choice for labor analgesia where we desire maintenance of maternal
mobility (“Push! Push!”).
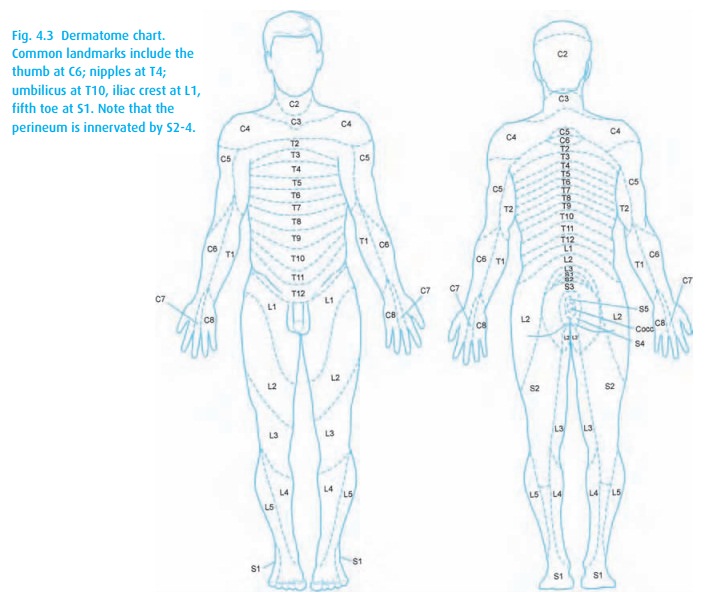
The
dermatomal level (Fig. 4.3) achieved depends
on several factors (Table4.2). Consider a Cesarean delivery, for which we require a T4
sensory level tominimize discomfort with uterine manipulation.
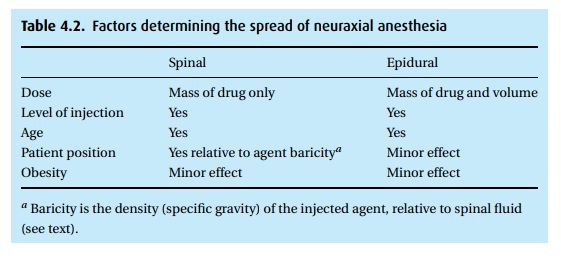
For an epidural, we select a local
anesthetic and concentration (e.g., 2% lidocaine with epinephrine), then
administer ∼5 mL boluses until we achieve the desired level (or we reach the
maximum dose allowed). For a spinal, we administer a calculated dose and then
use gravity to influence the level of the block.
Normal
cerebrospinal fluid (CSF) has a specific gravity (density relative to water) of
1.0006 ± 0.0003. Any agent of a different density,
injected into the CSF, will dis-tribute according to gravity. That is, a
hyperbaric agent will “sink,” and hypobaric
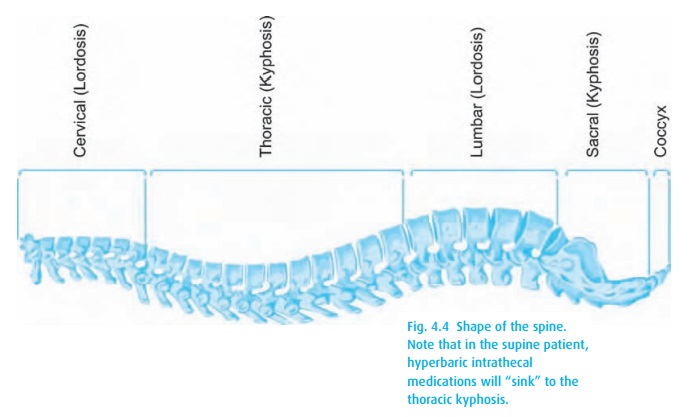
We can affect the resulting anesthetic level by tilting the patient.
To achieve a T4 level for our Cesarean delivery, we inject hyperbaric local
anesthetic (e.g., 12 mg of 0.75% bupivacaine with dextrose) intrathecally. When
the patient assumes a supine position, the local anesthetic “sinks” to the
thoracic kyphosis (Fig. 4.4). If, after a
few minutes, the level of the block remains too low, we can carefully lower the
patient’s head; as the drug follows gravity, the level will rise.
After
several minutes (the actual time depending on the agent selected), the drug
will be “fixed” and no further manipulation of its level can be achieved by
altering the patient’s position.
Hemodynamic effects
Unfortunately,
autonomic nerves (sympathetic here) are the easiest to block and cannot be
independently spared. The sympathetic block extends usually at least two
dermatome levels higher than the somatic sensory block. Basal sympathetic tone
causes vasoconstriction peripherally, thus its elimination results in
vaso-dilation (venous and arterial). Up to about a T4 level (nipple line),
hypotension results primarily from decreased preload secondary to vasodilation
proportionate to the sympathetic level (the higher the block, the more of the
peripheral vascula-ture escapes from nervous control and is “opened”). The
baroreflex response will attempt to maintain cardiac output. While its efforts
to vasoconstrict the blocked area are thwarted, vasoconstriction in the unblocked
area works overtime. Sym-pathetic stimulation reaches the heart via the
“cardiac accelerators,” which travel in T1–4 nerves; thus a higher block may
inhibit sympathetic stimulation of the heart, resulting in bradycardia and a
greater decrease of cardiac output and blood pressure.
Pulmonary effects
If the
neuraxial anesthesia level covers the thorax, intercostal muscle function will
be impaired. While not a problem for most patients, those who recruit acces-sory
muscles for normal breathing may have difficulty. Fortunately, the diaphragm
receives its innervation from C2–4, and therefore the neck should never be
affected by neuraxial anesthesia. If it is,
the block is much too high and the
patient will complain (if he still can) of dyspnea. Manual ventilation with bag
and mask will be required. Often, even tracheal intubation for maintenance of
the airway will become necessary. Yet, many patients become dyspneic at even a
mid-thoracic level of anesthesia, and usually without any decrease in their
oxyhemoglobin sat-uration. We attribute this to loss of chest wall
proprioception, which removes a feedback loop that reassures the patient’s
brain that ventilation is maintained. If the patient complains of shortness of
breath, first confirm that the level of anes-thesia is not too high. If
reassured on that point, let the patient put a hand in front of his mouth so
that he can feel his exhaled breath. This may restore the feed-back loop and
the patient’s sense of well being. If necessary, apply supplemental oxygen.
Complications
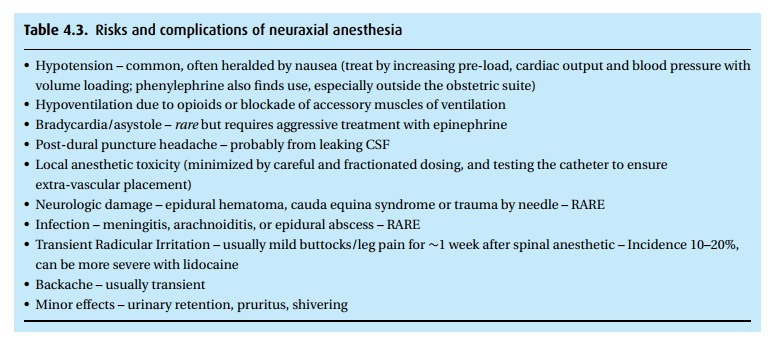
Of the
potential complications to neuraxial blockade (Table 4.3),
we fear forma-tion of an epidural hematoma most. Because the spinal cord runs
in the spinal canal, a closed space, anything that abnormally takes up room
causes compres-sion of other structures. Should an epidural blood vessel get
nicked on insertion of a needle (common), and that vessel fail to clot
normally, the resulting hematoma
For this
reason, patients who are anticoagulated or thrombocytopenic are rarely
consid-ered candidates for neuraxial blocks. This risk of epidural hematoma is
present both at insertion and removal
of the catheter.
Post-dural
puncture headache, another complication, deserves special men-tion: the patient
develops pounding headaches when sitting up and finds great relief by lying
down. A hole in the dura mater does not seal immediately. The size and shape of
that hole has implications for the future development of a post-dural puncture
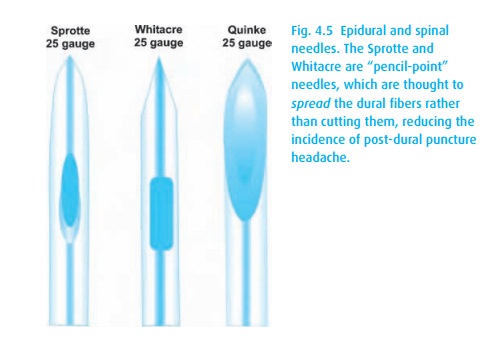
(spinal) headache. We can minimize the risk of this headache by using “pencil
point” needles (Fig. 4.5) in the smallest
diameter practical, e.g., 25–27 g. We do not use such small diameter needles
when performing a diagnostic lum-bar puncture, as it would take too long to
acquire fluid for laboratory studies. As you might imagine, post-dural puncture
headaches are particularly bad when we inadvertently nick the dura with the
large epidural needle1 during an
attempt to place an epidural catheter. This so-called “wet tap” has a high
incidence of headache, particularly in the pregnant patient. Treatment includes
bedrest, anal-gesics, intravenous caffeine, and an epidural blood patch in
which the patient’s own blood is sterilely injected into the epidural space,
causing usually immediate relief.
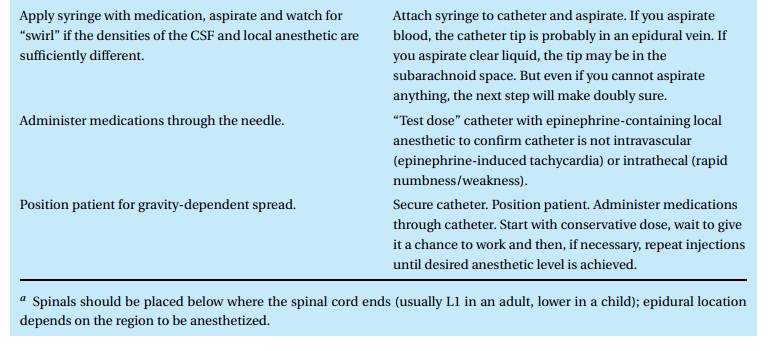
Technique
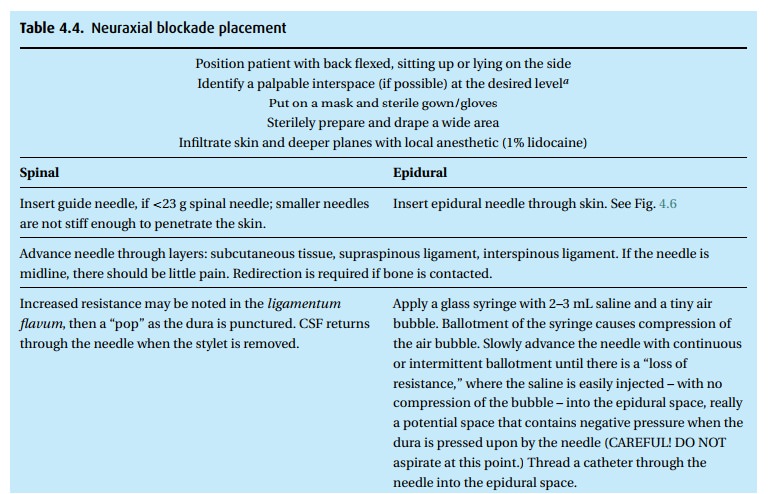
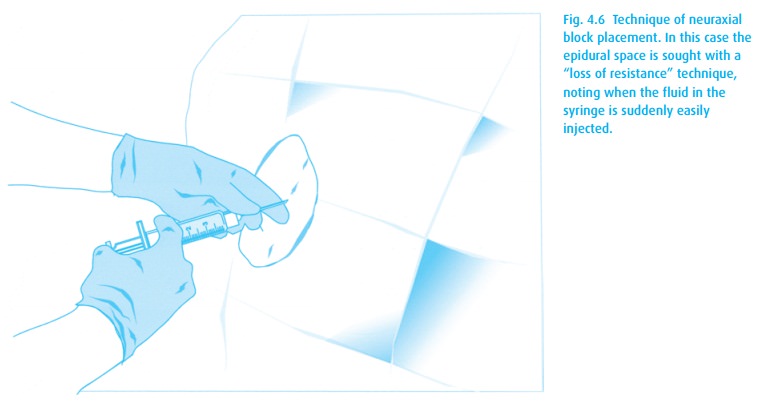
Neuraxial block placement requires both skill and the patient’s cooperation. Table 4.4lists the steps for placing either a spinal or epidural anesthetic. A com-bined spinal–epidural (CSE) begins as an epidural, but after identification of the epidural space with the epidural needle (Fig. 4.6), a spinal needle is passed through that needle and into the intrathecal space for injection of drug. The spinal needle is withdrawn, and the epidural catheter threaded as above.
Indications
Many
factors must be considered including location of operation and, therefore,
anesthetic level required, duration of surgery, and implications for
cardiovascular and respiratory function. For example, we would not use spinal
anesthesia in a patient in hemorrhagic shock or with significant aortic
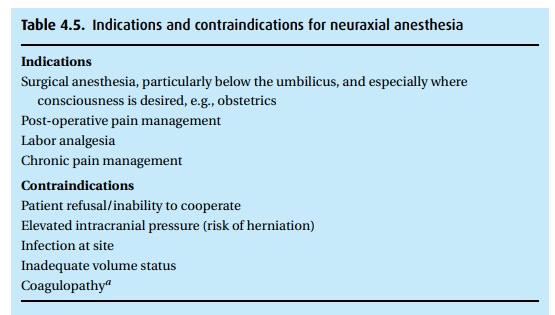
stenosis who would not tolerate a drop in preload and afterload (Table 4.5).
Related Topics