Chapter: Medical Physiology: Dietary Balances; Regulation of Feeding; Obesity and Starvation; Vitamins and Minerals
Neural Centers Regulate Food Intake
Neural Centers Regulate Food Intake
The sensation of hunger is associated with a craving for food and several other several physiologic effects, such as rhythmical contractions of the stomach and restlessness, which cause the person to search for an adequate food supply. A person’s appetite is a desire for food, often of a particular type, and is useful in helping to choose the quality of the food to be eaten. If the quest for food is successful, the feeling of satiety occurs. Each of these feelings is influenced by envi-ronmental and cultural factors, as well as by physio-logic controls that influence specific centers of the brain, especially the hypothalamus.
The Hypothalamus Contains Hunger and Satiety Centers.
Several neuronal centers of the hypothalamus partici-pate in the control of food intake. The lateral nuclei ofthe hypothalamus serve as a feeding center, and stimu-lation of this area causes an animal to eat voraciously (hyperphagia). Conversely, destruction of the lateralhypothalamus causes lack of desire for food and progressive inanition, a condition characterized by marked weight loss, muscle weakness, and decreased metabolism. The lateral hypothalamic feeding center operates by exciting the motor drives to search for food.
The ventromedial nuclei of the hypothalamus serveas the satiety center. This center is believed to give asense of nutritional satisfaction that inhibits the feeding center. Electrical stimulation of this region can cause complete satiety, and even in the presence of highly appetizing food, the animal refuses to eat (aphagia). Conversely, destruction of the ventromedialnuclei causes voracious and continued eating until the animal becomes extremely obese, sometimes as large as four times normal.
The paraventricular, dorsomedial, and arcuate nuclei of the hypothalamus also play a major role in regulat-ing food intake. For example, lesions of the paraven-tricular nuclei often cause excessive eating, whereas lesions of the dorsomedial nuclei usually depress eating behavior. As discussed later, the arcuate nuclei are the sites in the hypothalamus where multiple hor-mones released from the gastrointestinal tract and adipose tissue converge to regulate food intake as well as energy expenditure.
There is much chemical cross-talk among the neurons on the hypothalamus, and together, these centers coordinate the processes that control eating behavior and the perception of satiety. These nuclei of the hypothalamus also influence the secretion of several hormones that are important in regulating energy balance and metabolism, including those from the thyroid and adrenal glands, as well as the pancre-atic islet cells.
The hypothalamus receives neural signals from the gastrointestinal tract that provide sensory information about stomach filling, chemical signals from nutrients in the blood (glucose, amino acids, and fatty acids) that signify satiety, signals from gastrointestinal hormones, signals from hormones released by adipose tissue, and signals from the cerebral cortex (sight, smell, and taste) that influence feeding behavior. Some of these inputs to the hypothalamus are shown in Figure 71–1.
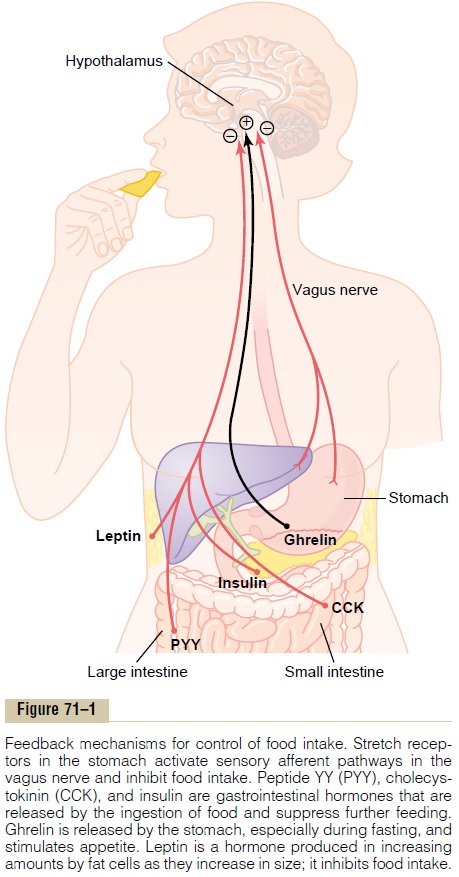
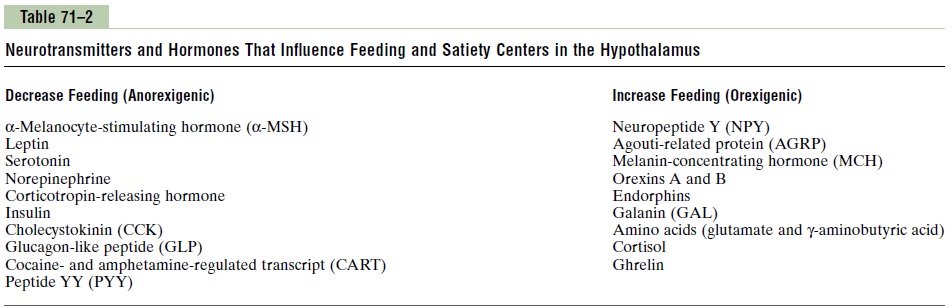
The hypothalamic feeding and satiety centers have a high density of receptors for neurotransmitters and hormones that influence feeding behavior. A few of the many substances that have been shown to alter appetite and feeding behavior in experimental studies are listed in Table 71–2 and are generally categorized as (1) orexigenic substances that stimulate feeding, or(2) anorexigenicsubstances that inhibit feeding.
Neurons and Neurotransmitters in the Hypothalamus That Stim-ulate or Inhibit Feeding. There are two distinct types ofneurons in the arcuate nuclei of the hypothalamus that are especially important as controllers of both appetite and energy expenditure (Figure 71–2): (1) pro-opiomelanocortin (POMC) neurons that producea-melanocyte-stimulating hormone (a-MSH) together with cocaine- and amphetamine-related transcript (CART), and (2) neurons that produce the orexigenicsubstances neuropeptide Y (NPY) and agouti-related protein (AGRP). Activation of the POMC neuronsdecreases food intake and increases energy expendi-ture, whereas activation of the NPY-AGRP neurons increases food intake and reduces energy expenditure.
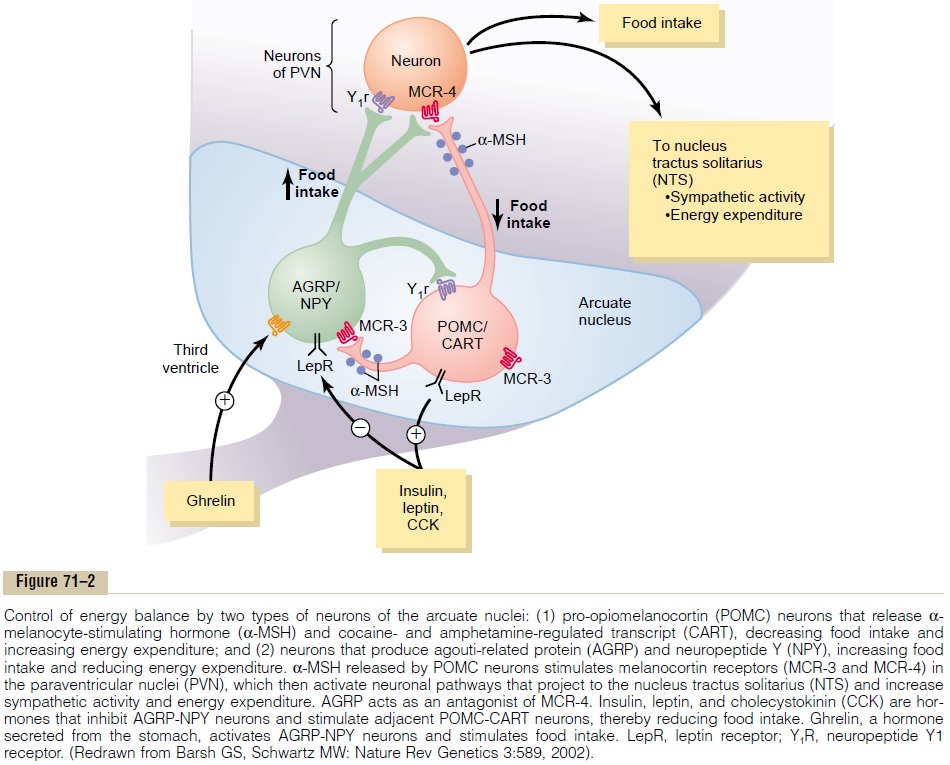
As discussed later, these neurons appear to be the major targets for the actions of several hormones that regulate appetite, includingleptin, insulin, cholecys-tokinin (CCK), and ghrelin. In fact, the neurons of thearcuate nuclei appear to be a site of convergence of many of the nervous and peripheral signals that regu-late energy stores.
The POMC neurons release a-MSH, which then acts on melanocortin receptors found especially in neurons of the paraventricular nuclei. Although there are at least five subtypes of melanocortin receptor (MCR), MCR-3 and MCR-4 are especially important in regulating food intake and energy balance. Activa-tion of these receptors reduces food intake while increasing energy expenditure. Conversely, inhibition of MCR-3 and MCR-4 greatly increases food intake and decreases energy expenditure. The effect of MCR activation to increase energy expenditure appears to be mediated, at least in part, by activation of neuronal pathways that project from the paraventricular nuclei to the nucleus tractus solitarius and stimulate sympa-thetic nervous system activity.
The hypothalamic melanocortin system plays a pow-erful role in regulating energy stores of the body, and defective signaling of the melanocortin pathway is associated with extreme obesity. In fact, mutations of MCR-4 represent the most common known mono-genic (single-gene) cause of human obesity, and some studies suggest that MCR-4 mutations may account for as much as 5 to 6 percentof early-onset severe obesity in children. In contrast, excessive activation of the melanocortin system reduces appetite. Some studies suggest that this activation may play a role in causing the anorexia associated with severe infections or cancer tumors.
AGRP released from the orexigenic neurons of the hypothalamus is a natural antagonist of MCR-3 and MCR-4 and probably increases feeding by inhibiting the effects of a-MSH to stimulate melanocortin recep-tors (see Figure 71–2). Although the role of AGRP in normal physiologic control of food intake is unclear, excessive formation of AGRP in mice and humans, due to gene mutations, is associated with excessive feeding and obesity.
NPY is also released from orexigenic neurons of the arcuate nuclei. When energy stores of the body are low, orexigenic neurons are activated to release NPY, which stimulates appetite. At the same time, firing of the POMC neurons is reduced, thereby decreasing the activity of the melanocortin pathway and further stim-ulating appetite.
Neural Centers That Influence the Mechanical Process of Feeding. Another aspect of feeding is the mechanicalact of the feeding process itself. If the brain is sec-tioned below the hypothalamus but above the mesen-cephalon, the animal can still perform the basic mechanical features of the feeding process. It can sali-vate, lick its lips, chew food, and swallow. Therefore, the actual mechanics of feeding are controlled by centers in the brain stem. The function of the othercenters in feeding, then, is to control the quantity of food intake and to excite these centers of feeding mechanics to activity.
Neural centers higher than the hypothalamus also play important roles in the control of feeding, partic-ularly in the control of appetite. These centers include the amygdala and the prefrontal cortex, which are closely coupled with the hypothalamus. It will be recalled from the discussion of the sense of smell that portions of the amygdala are a major part of the olfactory nervous system. Destructive lesions in the amygdala have demonstrated that some of its areas increase feeding, whereas others inhibit feeding. In addition, stimulation of some areas of the amygdala elicits the mechanical act of feeding. An important effect of destruction of the amygdala on both sides of the brain is a “psychic blindness” in the choice of foods. In other words, the animal (and pre-sumably the human being as well) loses or at least par-tially loses the appetite control that determines the type and quality of food it eats.
Related Topics