Chapter: Basic & Clinical Pharmacology : Vasoactive Peptides
Natriuretic Peptides
NATRIURETIC
PEPTIDES
Synthesis & Structure
The
atria and other tissues of mammals contain a family of pep-tides with
natriuretic, diuretic, vasorelaxant, and other properties. The family includes
atrial natriuretic peptide (ANP), brain natri-uretic peptide (BNP), and C-type
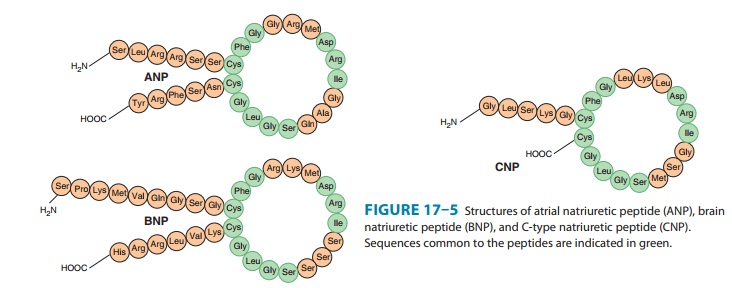
natriuretic peptide (CNP). The peptides share a common 17-amino-acid disulfide
ring with vari-able C- and N-terminals (Figure 17–5). A fourth peptide, urodila-tin,
has the same structure as ANP with an extension of four amino acids at the
N-terminal.
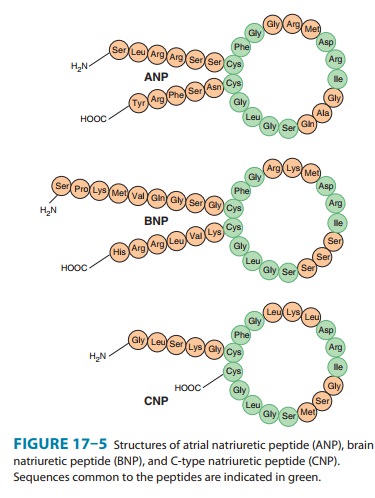
ANP
is derived from the carboxyl terminal end of a common precursor termed
preproANP. ANP is synthesized primarily in cardiac atrial cells, but it is also
synthesized in ventricular myocar-dium, by neurons in the central and
peripheral nervous systems, and in the lungs.
The
most important stimulus to the release of ANP from the heart is atrial stretch
via mechanosensitive ion channels. ANP release is also increased by volume
expansion, changing from the standing to the supine position, and exercise. ANP
release can also be increased by sympathetic stimulation via α1A adrenoceptors,
endothelins via the ETA-receptor subtype , glucocorti-coids, and
AVP. Plasma ANP concentration increases in several pathologic states, including
heart failure, primary aldosteronism, chronic renal failure, and inappropriate
ADH secretion syndrome.
Administration
of ANP produces prompt and marked increases in sodium excretion and urine flow.
Glomerular filtration rate increases, with little or no change in renal blood
flow, so that the filtration fraction increases. The ANP-induced natriuresis is
due to both the increase in glomerular filtration rate and a decrease in
proximal tubular sodium reabsorption. ANP also inhibits the release of renin,
aldosterone, and AVP; these changes may also increase sodium and water
excretion. Finally, ANP causes vasodi-lation and decreases arterial blood
pressure. Suppression of ANP production or blockade of its action impairs the
natriuretic response to volume expansion, and increases blood pressure.
BNP
was originally isolated from porcine brain but, like ANP, it is synthesized
primarily in the heart. It exists in two forms, hav-ing either 26 or 32 amino
acids (Figure 17–5). Like ANP, the release of BNP appears to be volume related;
indeed, the two peptides may be co-secreted. BNP exhibits natriuretic,
diuretic, and hypotensive activities similar to those of ANP but circulates at
a lower concentration.
CNP
consists of 22 amino acids (Figure 17–5). It is located predominantly in the
central nervous system but is also present in several other tissues including
the vascular endothelium, kidneys, and intestine. It has not been found in
significant concentrations in the circulation. CNP has less natriuretic and
diuretic activity than ANP and BNP but is a potent vasodilator and may play a
role in the regulation of peripheral resistance.
Urodilatin
is synthesized in the distal tubules of the kidneys by alternative processing
of the ANP precursor. It elicits potent natriure-sis and diuresis, and thus
functions as a paracrine regulator of sodium and water excretion. It also
relaxes vascular smooth muscle.
Pharmacodynamics & Pharmacokinetics
The
biologic actions of the natriuretic peptides are mediated through association
with specific high-affinity receptors located on the surface of the target
cells. Three receptor subtypes termed ANPA,ANPB,
and ANPC(also known
as NPR1, NPR2, and NPR3) havebeen identified.
The ANPA receptor consists of a 120 kDa mem-brane-spanning protein
with enzymatic activity associated with its intracellular domain. Its primary
ligands are ANP and BNP. The ANPB receptor is similar in structure
to the ANPA receptor, but its primary ligand appears to be CNP. The
ANPA and ANPB receptors, but not the ANPC
receptor, are guanylyl cyclase enzymes.
The
natriuretic peptides have a short half-life in the circula-tion. They are
metabolized in the kidneys, liver, and lungs by the neutral endopeptidase NEP
24.11. Inhibition of this endopepti-dase results in increases in circulating
levels of the natriuretic peptides, natriuresis, and diuresis. The peptides are
also removed from the circulation by binding to ANP C receptors in
the vascular endothelium. This receptor binds the natriuretic peptides with
equal affinity. The receptor and bound peptide are internalized, the peptide is
degraded enzymatically, and the receptor is returned to the cell surface.
Patients with heart failure have high plasma levels of ANP and BNP; the latter
has emerged as a diagnostic and prognostic marker in this condition.
Related Topics