Chapter: Modern Medical Toxicology: Hydrocarbons and Pesticides: Hydrocarbon
Naphthalene - Aromatic Hydrocarbons
Naphthalene
Synonyms
·
Moth flake, Tar camphor, White tar.
Physical Appearance
·
White scaly powder which volatilises
at room temperature.
Sources
·
Naphthalene occurs naturally in the
essential oils of the roots of Radix
and Herba ononidis, and crude oil.
·
It can be manufactured by crystallising and separating the
naphthalene fraction.
·
Naphthalene can also be produced by boiling coal tar oils at
temperatures between 200–2500 C, followed by crystal- lisation and
distillation.
·
It can also be derived from catalytic processing of
petro-leum, or isolated from cracked petroleum.
·
Naphthalene is formed in cigarette smoke by pyrolysis, and
is also a photodecomposition product of carbaryl, an agricultural pesticide.
Uses
·
Moth repellent (in the form of moth
balls) (Fig 27.2)*
·
Deodorant cakes
· Scintillation counters.

Toxicokinetics and Mode of Action
Naphthalene itself is not
responsible for the toxic effects. Its metabolites alpha and beta naphthol as
well as naphtho-quinone are powerful haemolytic agents. Individuals with
glucose-6-phosphate dehydrogenase (G- 6-PD) deficiency are especially vulnerable
to the toxicity of these metabolites. Naphthalene is first metabolised by
hepatic mixed func-tion oxidases to the epoxide, naphthalene-1,2-oxide. The
epoxide is enzymatically converted into the dihydrodiol, 1, 2-dihydroxy-
1,2-dihydronaphthalene or conjugated with glutathione. The dihydrodiol is then
conjugated to form a polar compound with glucuronic acid or sulfate, or further
dehy-drogenated to form highly reactive 1,2-dihydroxynaphthalene.
Dihydroxynaphthalene can be enzymatically conjugated with sulfate or glucuronic
acid or spontaneously oxidised to form 1,2-naphthoquinone. Naphthalene is also
metabolised to mercapturic acid derivatives.
Naphthalene metabolites (naphthols
and naphthylglycuro-nates) are excreted in the urine as 1-naphthylmercapturic
acid (15% of absorbed dose), as conjugates of 1,2-dihydronaphtha-lene-1,2-diol
(10% of absorbed dose), and as 1- and 2-naphthols and 1,2-dihydroxynaphthalene.
Conjugates of glutathione (cysteinylglycine, and cysteine; intermediates in
formation of mercapturic acids) are excreted mainly in the bile as metabolites
of naphthalene.
Naphthalene can be absorbed via
oral, inhalation, and dermal routes.
Clinical Features
·
Non-haemolytic manifestations: Vomiting, abdominal
pain,diarrhoea, headache, diaphoresis, optic neuritis, restlessness, lethargy,
fever, convulsions, hepatomegaly, splenomegaly. Hyperbilirubinaemia and fatal
kernicterus may occur in newborns with significant haemolysis. Centrilobular
necrosis occurred in one paediatric poisoning case. Coma and acute lung injury
may develop in severe toxicity. Naphthalene skin exposure may cause
hypersensitivity dermatitis. Repeated exposure may cause corneal ulceration,
lenticular opacities, cataracts, headache, malaise and vomiting.
·
Haemolytic manifestations: Pallor, weakness,
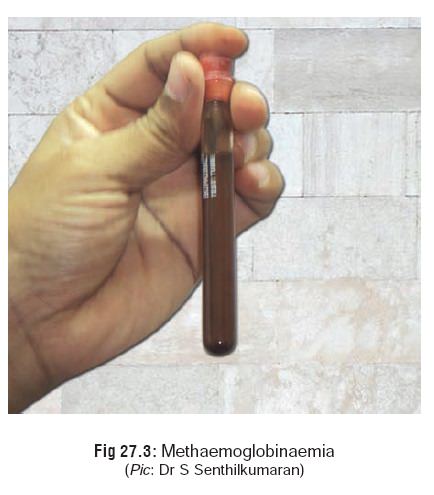
jaundice,cyanosis, haemolysis, haemolytic anaemia, methaemoglo-binaemia (Fig 27.3), hyperkalaemia, dysuria,
haematuria, and dark urine (haemoglobinuria), albuminuria, oliguria, and acute
renal failure. Cardiovascular shock can occur in patients with severe
haemolytic anaemia. Metabolic acidosis may develop in patients with acute renal
failure secondary to haemolysis.
o Haematological Findings: Increased
WBC count, fragmented RBC, anisocytosis, Heinz bodies, and poikilocytosis.
o Infants and patients with G6PD deficiency, sickle cell anaemia, or sickle cell trait are more likely to develop haemolysis and/or methaemoglobinaemia.
·
Chronic exposure to naphthalene can result in aplastic anaemia,
hepatic necrosis, and jaundice. Naphthalene and coal tar exposure have been
associated with laryngeal and intestinal carcinoma.
Diagnosis
·
Obtain baseline CBC, electrolytes, glucose-6-phosphate
dehydrogenase level, liver enzymes and renal function tests, urinalysis and
urine dipstick test for haemoglobinuria.
·
Measurement of urinary metabolites (1-naphthol or
mercap-turic acid) may help to confirm the diagnosis. Urinary naphthol levels
may be utilised to monitor industrial creosote exposure (naphthalene is the
most abundant compound found in creosote vapour).
·
X-ray: Abdominal radiographs may help differentiate between
mothballs or other products which contain paradi-chlorobenzene (densely
radiopaque) from those which contain naphthalene (radiolucent or faintly
radiopaque).
Treatment
Acute exposure is treated on the
same lines as in the case of aliphatic hydrocarbons.
·
Ingestion of one mothball may produce toxicity; patients
with ingestion of more than this amount should be referred to a health care
facility for gastric decontamination and observation. If laboratory findings
are negative and the patient is asymptomatic during a 4 to 6-hour observation
period, the patient may be discharged with instructions to return for a
follow-up CBC and urinalysis for up to 5 days post-ingestion. Patients should
be instructed to return if any gastrointestinal symptoms, pallor, dark or
diminished urine output, or CNS symptoms develop.
· Decontamination—
o Induced emesis is more useful for
mothballs because of their size. Do not induce vomiting if the patient has any
evidence of lethargy or CNS depression.
o Gastric lavage may be useful for
ingestion of flakes, but its effectiveness may be limited by naphthalene’s poor
water solubility. Mothballs dissolve slowly; gastric decontamination should be
considered even in patients presenting late after ingestion.
o Information on the benefit of
activated charcoal is scant, but adsorption is thought to occur. Consider
adminis-tration of activated charcoal after a potentially toxic ingestion (up
to 1 hour).
o Dermal Decontamination—Remove
contaminated clothing and wash exposed area thoroughly with soap and water.
Consider discarding contaminated clothing, as washing does not easily remove
naphthalene.
·
Avoid oral administration of oil or fatty substances.
·
Control seizures.
·
Alkaline Diuresis—
o Should be performed if there is
evidence of haemolysis. This may prevent renal deposition of red blood cell
break down products in the renal tubules and resultant renal failure.
o Administer 1 to 2 mEq/kg of sodium
bicarbonate as an intravenous bolus. Add 132 mEq (3 ampoules) sodium
bicarbonate and 20 to 40 mEq potassium chloride (as needed) to one litre of
dextrose 5% in water and infuse at approximately 1.5 times the maintenance
fluid rate.
o In patients with underlying
dehydration additional administration of 0.9% saline may be needed to main-tain
adequate urine output (1 to 2 ml/kg/hour).
o Manipulate bicarbonate infusion to
maintain a urine pH of at least 7.5. Additional sodium bicarbonate (1 to 2
mEq/kg) and potassium chloride (20 to 40 mEq/L) may be needed to achieve an
alkaline urine. Do not administer potassium to an oliguric or anuric patient.
o Obtain hourly intake/output and
urine pH. Assure adequate hydration and renal function prior to
alkalini-sation. Monitor fluid and electrolyte balance carefully.
o Monitor blood pH, especially in
intubated patients, to avoid severe alkalaemia. Administer furosemide as needed
to maintain urine output.
·
Treat haemolysis with blood transfusion, packed red cell
transfusions, or exchange transfusion.
·
Monitor methaemoglobin level and treat if symptomatic, or if
methaemoglobin levels are greater than 30%. Treat with methylene blue 1 to 2
mg/kg/dose (0.1 to 0.2 ml/kg/dose) IV over 5 minutes as needed every 4 hours.
It is important to remember that large doses of methylene blue may itself cause
methaemoglobinaemia or haemolysis. Also, meth-ylene blue must not be
administered if the patient has G6PD deficiency.
·
Haemodialysis may help enhance elimination, though it is not
routinely recommended.
Forensic Issues
·
Most cases of exposure are accidental in nature.
·
A few are suicidal.
·
In the case of mothball ingestion (suicidal or accidental),
sometimes there is confusion as to whether the active ingredient is
naphthalene, camphor or paradichlorobenzene.
Differentiating between mothballs
containing paradi- chlorobenzene (PDB), naphthalene and camphor:
––
Physical appearance—Naphthalene is dry, while PDB has a wet and oily
appearance.
––Specific gravity—Distinguishing between
camphor, naphthalene, and PDB mothballs can be done by testing whether they
float or sink in a saturated solu- tion of salt water (4 ounces of tepid water
to which 3 heaping tablespoons of table salt has been added and stirred
vigorously until the salt will not dissolve any more). Camphor mothballs float
in both water and salt solution. Naphthalene mothballs sink in water but float
in saturated salt solution. PDB mothballs sink in both water and salt solution.
Solubility—PDB
is more soluble in turpentine than naphthalene. A mothball of PDB will usually
dissolve within 30 to 60 minutes whereas at least one fourth of the naphthalene
will be left.
Heat—PDB
produces a bright green colour in a bunsen burner flame; Naphthalene does not.
Melting point—PDB:
530 C; Naphthalene: 800 C. Placing
a small piece of the mothball in a test tubeheated to 600
C in water bath may simplify the melting point test. PDB will liquefy within
several minutes; naphthalene will remain intact.
Chemical test—If
chloroform and ammonium chlo-ride powder are added to PDB no colour change
occurs; naphthalene turns blue.
Related Topics