Chapter: 10th Science : Chapter 8 : Periodic Classification of Elements
Modern Periodic Table
MODERN PERIODIC TABLE
With reference to the
modern periodic law, the elements were arranged in the increasing order of
their atomic numbers to form the modern periodic table. The modern periodic
table is a tabular arrangement of elements in rows and columns, highlighting
the regular repetition of properties of the elements. Figure 8.1 shows the
modern periodic table of 118 elements discovered so far.![]()
![]()
As you have studied the
features of the modern periodic table in standard IX, here let us confine to
the study of the features of periods and groups.
1. Features of Periods
The horizontal rows are
called periods. There are seven periods in the periodic table.
First period (Atomic number 1 and 2):
This is the shortest period. It contains only two elements (Hydrogen and
Helium).
Second period (Atomic number 3 to 10):
This is a short period. It contains eight elements (Lithium to Neon).
Third period (Atomic number 11 to
18): This is also a short period. It contains eight elements (Sodium to
Argon).
Fourth period (Atomic number 19 to
36): This is a long period. It contains eighteen elements (Potassium to
Krypton). This includes 8 normal elements and 10 transition elements.
Fifth period (Atomic number 37 to
54): This is also a long period. It contains 18 elements (Rubidium to
Xenon). This includes 8 normal elements and 10 transition elements.
Sixth period (Atomic number 55 to
86): This is the longest period. It contains 32 elements (Caesium to
Radon). This includes 8 normal elements, 10 transition elements and 14 inner
transition elements (Lanthanides).
Seventh period (Atomic number 87 to
118): Like the sixth period, this period also accommodates 32 elements.
Recently 4 elements have been included by IUPAC.

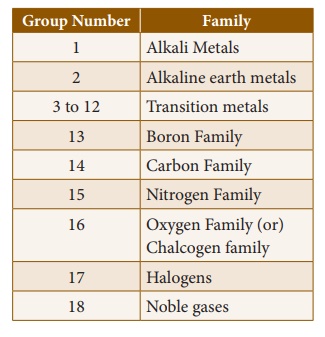
2. Features of Groups
The vertical columns in
the periodic table starting from top to bottom are called groups. There
are 18 groups in the periodic table.
Based on the common
characteristics of elements in each group, they can be grouped as various
families.
The Lanthanides and
Actinides, which form part of Group 3 are called inner transition
elements.
Except 'group 0', all
the elements present in each group have the same number of electrons in their
valence shell and thus have the same valency. For example, all the elements of
group 1 have one electron in their valence shells (1s1). So, the valency of all
the alkali metals is ‘1’.
As the elements present
in a group have identical valence shell electronic configurations, they possess
similar chemical properties.
The physical properties
of the elements in a group such as melting point, boiling point and density
vary gradually.
The atoms of the 'group
0' elements have stable electronic configuration in their valence shells and
hence they are unreactive.
Related Topics