Chapter: Basic & Clinical Pharmacology : The Gonadal Hormones & Inhibitors
Metabolism - The Testis
METABOLISM
In many target
tissues, testosterone is converted to dihydrotestos-terone by 5α-reductase. In these
tissues, dihydrotestosterone is the major active androgen. The conversion of
testosterone to estradiol by P450 aromatase also occurs in some tissues,
including adipose tissue, liver, and the hypothalamus, where it may be of
importance in regulating gonadal function.
The major
pathway for the
degradation of testosterone
in humans occurs in the liver, with the reduction of the double bond and
ketone in the A ring, as is seen in other steroids with a 4-ketone
configuration in the A ring. This leads to the produc-tion of
inactive substances such
as androsterone and
etiocholanolone that are then conjugated and excreted in the urine.
Androstenedione,
dehydroepiandrosterone (DHEA), and dehydroepiandrosterone sulfate (DHEAS) are
also produced in significant amounts in humans, although largely in the adrenal
gland rather than in the testes. They contribute slightly to the normal
maturation process supporting other androgen-dependent pubertal changes in the
human, primarily development of pubic and axillary hair and bone maturation. As
noted, some studies suggest that DHEA and DHEAS may have other central nervous
system and metabolic effects and may prolong life in rabbits. In men they may
improve the sense of well-being and inhibit atherosclerosis. In a
placebo-controlled clinical trial in patients with systemic lupus
erythematosus, DHEA demonstrated some beneficial effects (see Adrenal
Androgens). Adrenal androgens are to a large extent metabolized in the same
fashion as testosterone. Both steroids—but particularly androstenedione— can be
converted by peripheral tissues to estrone in very small amounts (1–5%). The
P450 aromatase enzyme responsible for this conversion is also found in the
brain and is thought to play an important role in development.
Physiologic Effects
In the normal male,
testosterone or its active metabolite 5α-dihydrotestosterone is responsible for the
many changes that occur in puberty. In addition to the general growth-promoting
properties of androgens on body tissues, these hormones are responsible for
penile and scrotal growth. Changes in the skin include the appearance of pubic,
axillary, and beard hair. The sebaceous glands become more active, and the skin
tends to become thicker and oilier. The larynx grows and the vocal cords become
thicker, leading to a lower-pitched voice. Skeletal growth is stimulated and
epiphysial closure accelerated. Other effects include growth of the prostate
and seminal vesicles, darkening of the skin, and increased skin circulation.
Androgens play an impor-tant role in stimulating and maintaining sexual
function in men. Androgens increase lean body mass and stimulate body hair
growth and sebum secretion. Metabolic effects include the reduc-tion of hormone
binding and other carrier proteins and increased liver synthesis of clotting
factors, triglyceride lipase, α1-antitrypsin, haptoglobin, and sialic acid.
They also stimulate renal erythropoi-etin secretion and decrease HDL levels.
Synthetic Steroids with Androgenic & Anabolic Action
Testosterone, when
administered by mouth, is rapidly absorbed. However, it is largely converted to
inactive metabolites, and only about one sixth of the dose administered is
available in active form. Testosterone can be administered parenterally, but it
has a more prolonged absorption time and greater activity in the propionate,
enanthate, undecanoate, or cypionate ester forms. These derivatives are
hydrolyzed to release free testosterone at the site of injection. Testosterone
derivatives alkylated at the 17 position, eg, methyltes-tosterone and
fluoxymesterone, are active when given by mouth.
Testosterone and its
derivatives have been used for their ana-bolic effects as well as in the
treatment of testosterone deficiency.
Although
testosterone and other known active steroids can be isolated in pure form and
measured by weight, biologic assays are still used in the investigation of new
compounds. In some of these studies in animals, the anabolic effects of the
compound as measured by trophic effects on muscles or the reduction of nitrogen
excretion may be dissociated from the other androgenic effects. This has led to
the marketing of compounds claimed to have anabolic activity associated with
only weak androgenic effects. Unfortunately, this dissociation is less marked
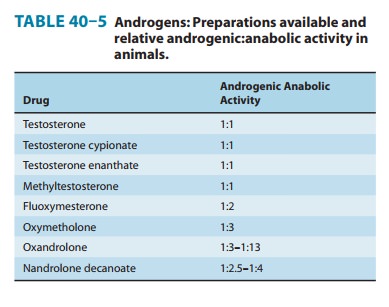
in humans than in the animals used for testing (Table 40–5), and all are potent
androgens.
Pharmacologic Effects
A. Mechanism of Action
Like other steroids,
testosterone acts intracellularly in target cells. In skin, prostate, seminal
vesicles, and epididymis, it is converted to 5α-dihydrotestosterone by 5α-reductase. In these
tissues, dihy-drotestosterone is the dominant androgen. The distribution of
this enzyme in the fetus is different and has important developmental
implications.
Testosterone
and dihydrotestosterone bind to the intracellular androgen receptor, initiating
a series of events similar to those described above for estradiol and
progesterone, leading to growth, differentiation, and synthesis of a variety of
enzymes and other functional proteins.
B. Effects
In the male at puberty, androgens cause development of the sec-ondary sex characteristics (see above). In the adult male, large doses of testosterone—when given alone—or its derivatives sup-press the secretion of gonadotropins and result in some atrophy of the interstitial tissue and the tubules of the testes. Since fairly large doses of androgens are required to suppress gonadotropin secre-tion, it has been postulated that inhibin, in combination with androgens, is responsible for the feedback control of secretion. In women, androgens are capable of producing changes similar to those observed in the prepubertal male. These include growth of facial and body hair, deepening of the voice, enlargement of the clitoris, frontal baldness, and prominent musculature. The natural androgens stimulate erythrocyte production.
The administration of
androgens reduces the excretion of nitro-gen into the urine, indicating an
increase in protein synthesis or a decrease in protein breakdown within the
body. This effect is much more pronounced in women and children than in normal
men.
Clinical Uses
A. Androgen Replacement Therapy in Men
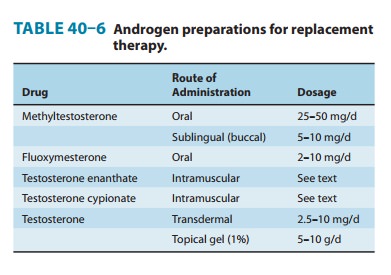
Androgens are used to
replace or augment endogenous androgen secretion in hypogonadal men (Table
40–6). Even in the presence of pituitary deficiency, androgens are used rather
than gonadotropin except when normal spermatogenesis is to be achieved. In
patients with hypopituitarism, androgens are not added to the treatment regimen
until puberty, at which time they are instituted in gradually increasing doses
to achieve the growth spurt and the development of secondary sex
characteristics. In these patients, therapy should be started with long-acting
agents such as testosterone enanthate or cypionate in doses of 50 mg
intramuscularly, initially every 4, then every 3, and finally every 2 weeks,
with each change taking place at 3-month intervals. The dose is then doubled to
100 mg every 2 weeks until maturation is complete. Finally, it is changed to
the adult replacement dose of 200 mg at 2-week intervals.
Testosterone
propionate, though potent, has a short duration of action and is not practical
for long-term use. Testosterone unde-canoate can be given orally, administering
large amounts of the steroid twice daily (eg, 40 mg/d); however, this is not
recom-mended because oral testosterone administration has been associ-ated with
liver tumors. Testosterone can also be administered transdermally; skin patches
or gels are available for scrotal or other skin area application. Two
applications daily are usually required for replacement therapy. Implanted
pellets and other longer-acting preparations are under study. The development
of polycythemia or hypertension may require some reduction in dose.
B. Gynecologic Disorders
Androgens are used
occasionally in the treatment of certain gyne-cologic disorders, but the
undesirable effects in women are suchthat they must be used with great caution.
Androgens have been used to reduce breast engorgement during the postpartum
period, usually in conjunction with estrogens. The weak androgen danazol is
used in the treatment of endometriosis (see above).
Androgens are
sometimes given in combination with estrogens for replacement therapy in the
postmenopausal period in an attempt to eliminate the endometrial bleeding that
may occur when only estrogens are used and to enhance libido. They have been
used for chemotherapy of breast tumors in premenopausal women.
C. Use as Protein Anabolic Agents
Androgens and anabolic
steroids have been used in conjunction with dietary measures and exercises in
an attempt to reverse pro-tein loss after trauma, surgery, or prolonged
immobilization and in patients with debilitating diseases.
D. Anemia
In
the past, large doses of androgens were employed in the treatment of refractory
anemias such as aplastic anemia, Fanconi’s anemia, sickle cell anemia,
myelofibrosis, and hemolytic anemias. Recombinant erythropoietin has largely
replaced androgens for this purpose.
E. Osteoporosis
Androgens and anabolic
agents have been used in the treatment of osteoporosis, either alone or in
conjunction with estrogens. With the exception of substitution therapy in
hypogonadism, bisphos-phonates have largely replaced androgen use for this
purpose.
F. Use as Growth Stimulators
These agents have been
used to stimulate growth in boys with delayed puberty. If the drugs are used
carefully, these children will probably achieve their expected adult height. If
treatment is too vigorous, the patient may grow rapidly at first but will not achieve
full predicted final stature because of the accelerated epiphysial closure that
occurs. It is difficult to control this type of therapy adequately even with
frequent X-ray examination of the epiphyses, since the action of the hormones
on epiphysial centers may con-tinue for many months after therapy is
discontinued.
G. Anabolic Steroid and Androgen Abuse in Sports
The use of anabolic
steroids by athletes has received worldwide attention. Many athletes and their
coaches believe that anabolic steroids—in doses 10–200 times larger than the
daily normal physiologic production—increase strength and aggressiveness,
thereby improving competitive performance. Such effects have been unequivocally
demonstrated only in women. Furthermore, the adverse effects of these drugs
clearly make their use inadvisable.
H. Aging
Androgen production
falls with age in men and may contribute to the decline in muscle mass,
strength, and libido. Preliminary studies of androgen replacement in aging
males with low andro-gen levels show an increase in lean body mass and
hematocrit and a decrease in bone turnover. Longer studies will be required to
assess the usefulness of this therapy.
Adverse Effects
The adverse effects of
these compounds are due largely to their masculinizing actions and are most
noticeable in women and pre-pubertal children. In women, the administration of
more than 200–300 mg of testosterone per month is usually associated with
hirsutism, acne, amenorrhea, clitoral enlargement, and deepening of the voice.
These effects may occur with even smaller doses in some women. Some of the
androgenic steroids exert progesta-tional activity, leading to endometrial
bleeding upon discontinua-tion. These hormones also alter serum lipids and
could conceivably increase susceptibility to atherosclerotic disease in women.
Except under the most
unusual circumstances, androgens should not be used in infants. Recent studies
in animals suggest that administration of androgens in early life may have
profound effects on maturation of central nervous system centers governing
sexual development, particularly in the female. Administration of these drugs
to pregnant women may lead to masculinization or undermasculinization of the
external genitalia in the female and male fetus, respectively. Although the
above-mentioned effects may be less marked with the anabolic agents, they do
occur. Sodium retention and edema are not common but must be
carefully watched for in patients with heart and kidney disease.
Most
of the synthetic androgens and anabolic agents are 17-alkyl-substituted
steroids. Administration of drugs with this structure is often associated with
evidence of hepatic dysfunction. Hepatic dysfunction usually occurs early in
the course of treat-ment, and the degree is proportionate to the dose.
Bilirubin levels may increase until clinical jaundice is apparent. The
cholestatic jaundice is reversible upon cessation of therapy, and permanent
changes do not occur. In older males, prostatic hyperplasia may develop,
causing urinary retention.
Replacement therapy in
men may cause acne, sleep apnea, erythrocytosis, gynecomastia, and azoospermia.
Supraphysiologic doses of androgens produce azoospermia and decrease in
testicular size, both of which may take months to recover after cessation of
therapy. The alkylated androgens in high doses can produce pelio-sis hepatica,
cholestasis, and hepatic failure. They lower plasma HDL and may increase LDL.
Hepatic adenomas and carcinomas have also been reported. Behavioral effects
include psychological dependence, increased aggressiveness, and psychotic
symptoms.
Contraindications & Cautions
The use of androgenic
steroids is contraindicated in pregnant women or women who may become pregnant
during the course of therapy.
Androgens should not
be administered to male patients with carcinoma of the prostate or breast.
Until more is known about the effects of these hormones on the central nervous
system in develop-ing children, they should be avoided in infants and young
children.
Special caution is
required in giving these drugs to children to produce a growth spurt. In most
patients, the use of somatotropin is more appropriate .
Care
should be exercised in the administration of these drugs to patients with renal
or cardiac disease predisposed to edema. If sodium and water retention occurs,
it will respond to diuretic therapy.
Methyltestosterone
therapy is associated with creatinuria, but the significance of this finding is
not known.
Caution: Several cases of
hepatocellular carcinoma have beenreported in patients with aplastic anemia
treated with androgen anabolic therapy. Erythropoietin and colony-stimulating
factors should be used instead.
Related Topics