Chapter: Basic & Clinical Pharmacology : Adrenoceptor Agonists & Sympathomimetic Drugs
Medicinal Chemistry of Sympathomimetic Drugs
MEDICINAL
CHEMISTRY OF SYMPATHOMIMETIC DRUGS
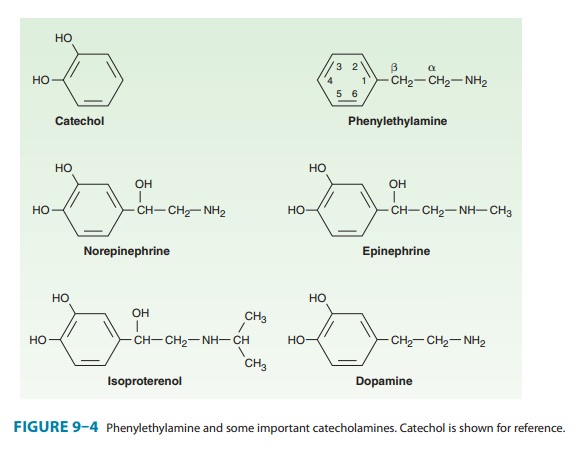
Phenylethylamine
may be considered the parent compound from which sympathomimetic drugs are
derived (Figure 9–4). This compound consists of a benzene ring with an
ethylamine side chain. Substitutions may be made on (1) the benzene ring, (2)
the terminal amino group, and (3) the α orβ carbons of the ethylamino chain. Substitution
by –OH groups at the 3 and 4 positions yields sympathomimetic drugs
collectively known as catecholamines. The effects of modification of
phenylethylamine are to change the affinity of the drugs for α and β receptors, spanning
the range from almost pure α activity (methoxamine) to almost pure β activity
(isoproterenol), as well as to influence the intrinsic ability to activate the
receptors.
In
addition to determining relative affinity to receptor subtype, chemical
structure also determines the pharmacokinetic properties and bioavailability of
these molecules.
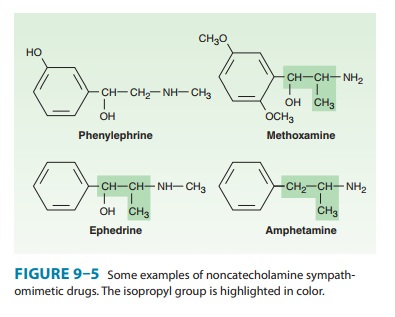
A.Substitution on the Benzene Ring
Maximal
α
and β
activity is found with catecholamines, ie, drugs having –OH groups at the 3 and
4 positions on the benzene ring. The absence of one or the other of these
groups, particularly the hydroxyl at C3, without other substitutions
on the ring may dra-matically reduce the potency of the drug. For example,
phe-nylephrine (Figure 9–5) is much less potent than epinephrine; indeed, α-receptor affinity is
decreased about 100-fold and β activity is almost negligible except at very
high concentrations. On the other hand, catecholamines are subject to
inactivation by catechol- O-methyltransferase
(COMT), and because this enzyme is found in the gut and liver, catecholamines
are not active orally . Absence of one or both –OH groups on the phe-nyl ring
increases the bioavailability after oral administration and prolongs the
duration of action. Furthermore, absence of ring –OH groups tends to increase
the distribution of the molecule to the central nervous system. For example,
ephedrine and amphet-amine (Figure 9–5) are orally active, have a prolonged
duration of action, and produce central nervous system effects not typically
observed with the catecholamines.
B. Substitution on the Amino Group
Increasing
the size of alkyl substituents on the amino group tends to increase β-receptor activity.
For example, methyl substitution on norepinephrine, yielding epinephrine,
enhances activity at β2 receptors. Beta activity is further enhanced
with isopropyl substi-tution at the amino group (isoproterenol). Beta2-selective
agonists generally require a large amino substituent group. The larger the
substituent on the amino group, the lower the activity at α recep-tors; for
example, isoproterenol is very weak at α receptors.
C. Substitution on the Alpha Carbon
Substitutions at the α carbon block oxidation by monoamine oxi-dase (MAO) and prolong the action of such drugs, particularly the noncatecholamines.
Ephedrine and amphetamine are examples of α-substituted compounds (Figure 9–5).
Alpha-methyl com-pounds are also called phenylisopropylamines. In addition to
their resistance to oxidation by MAO, some phenylisopropylam-ines have an
enhanced ability to displace catecholamines from storage sites in noradrenergic
nerves . Therefore, a portion of their activity is dependent on the presence of
normal norepinephrine stores in the body; they are indirectly acting
sympathomimetics.
D. Substitution on the Beta Carbon
Direct-acting
agonists typically have a β-hydroxyl group, although dopamine does not.
In addition to facilitating activation of adre-noceptors, this hydroxyl group
may be important for storage of sympathomimetic amines in neural vesicles.
Related Topics