Kinetic Theory of Gases - Law of Equipartition of Energy | 11th Physics : UNIT 9 : Kinetic Theory of Gases
Chapter: 11th Physics : UNIT 9 : Kinetic Theory of Gases
Law of Equipartition of Energy
LAW OF
EQUIPARTITION OF ENERGY
We
have seen in Section 9.2.1 that the average kinetic energy of a molecule moving
in x direction is
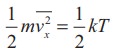
Similarly,
when the motion is in y direction,

According to kinetic theory, the
average kinetic energy of system of molecules in thermal equilibrium at
temperature T is uniformly distributed to all degrees of freedom (x or y or z
directions of motion) so that each degree of freedom will get 1/2 kT of energy.
This is called law of equipartition of energy.
Average
kinetic energy of a monatomic molecule (with f=3) =
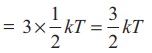
Average
kinetic energy of diatomic molecule at low temperature (with f = 5)
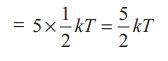
Average
kinetic energy of a diatomic molecule at high temperature (with f =7)
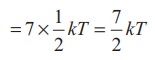
Average
kinetic energy of linear triatomic molecule (with f = 7) =
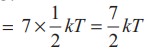
Average
kinetic energy of nonlinear tri atomic molecule (with f = 6) =
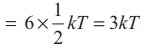
Application of law of equipartition energy in specific heat of a gas
Meyer’s
relation CP − CV = R connects the two specific heats for
one mole of an ideal gas.
Equipartition
law of energy is used to calculate the value of CP − CV
and the ratio between them γ = CP / CV.
Here
γ is called adiabatic exponent.
i) Monatomic molecule
Average
kinetic energy of a molecule
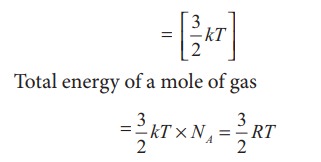
For
one mole, the molar specific heat at constant volume
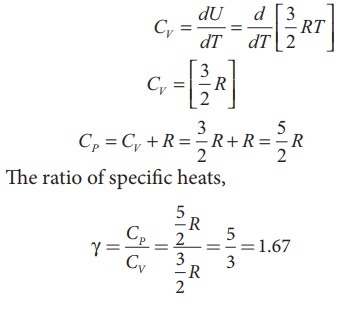
ii) Diatomic molecule
Average kinetic energy of a diatomic molecule at low temperature = 5/2kT
Total
energy of one mole of gas
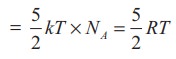
(Here,
the total energy is purely kinetic)
For
one mole Specific heat at constant volume
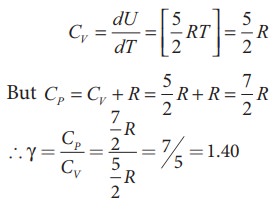
Energy
of a diatomic molecule at high temperature is equal to 7/2RT
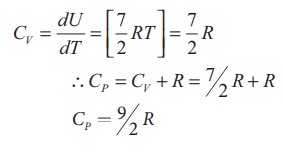
Note
that the CV and CP are higher for diatomic
molecules than the mono atomic molecules. It implies that to increase the
temperature of diatomic gas molecules by 1°C it require more heat energy than
monoatomic molecules.
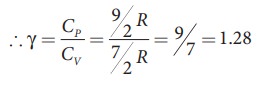
iii) Triatomic molecule
a) Linear molecule
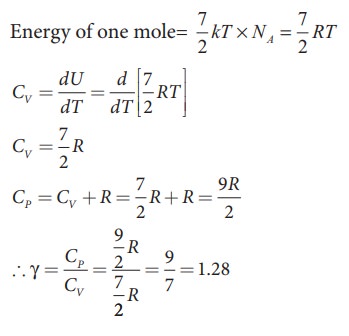
b) Non-linear molecule
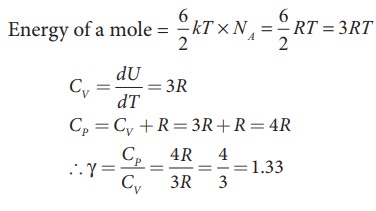
Note
that according to kinetic theory model of gases the specific heat capacity at
constant volume and constant pressure are independent of temperature. But in
reality it is not sure. The specific heat capacity varies with the temperature.
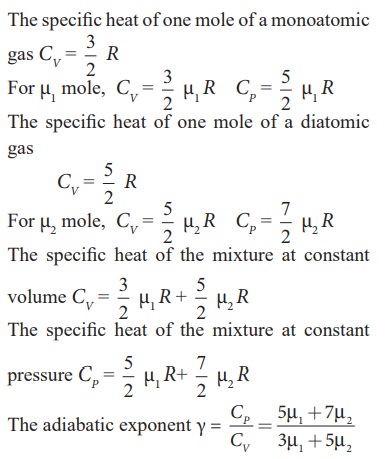
EXAMPLE 9.5
Find
the adiabatic exponent γ for mixture of μ 1 moles
of monoatomic gas and μ2 moles of a diatomic gas at normal
temperature.
Solution
Related Topics