Chapter: The Diversity of Fishes: Biology, Evolution, and Ecology: Functional morphology of locomotion and feeding
Jaw protrusion: the great leap forward - Fishes
Jaw protrusion: the great leap forward
Jaws evolved in fishes. The major difference between vertebrates and invertebrates is not so much the development of an ossified and constricted backbone; coelacanths, lungfishes, and sturgeons all lack distinct vertebral centra. Thereal advance that undoubtedly drove vertebrate evolution was the assembly of closable jaws used in feeding. The mechanics of jaw function and adaptive variation in jaw elements tell us a great deal about both how fishes feed and how fishes evolved.
As will be discussed, one of the major advances made by, but not exclusive to, higher teleost’s isthe ability to protrude the upper jaw during feeding. Jaw protrusion makes possible the pipette mouth of the higherteleost’s. Pipetting creates suction forces that can pull items from as far away as 25–50% of head length. Jaw protrusion also functions to overtake a prey item, extending the food-getting apparatus around the prey faster than the predator can move its entire body through the water. Attack velocity may thus be increased by up to 40%. As many as15 different functions and advantages have been postulated for the protrusible jaw of teleost’s. These advantages generally involve increased prey capture ability and efficiency but also suggest that antipredator surveillance and escape ability may be enhanced (Lauder & Liem 1981; Motta 1984;Ferry-Graham & Lauder 2001).
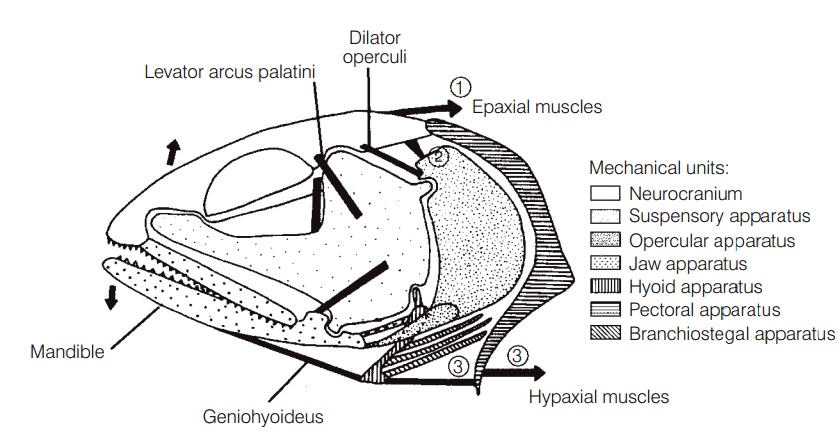
The elements involved in jaw protrusion include the bones of the jaw (premaxilla, maxilla, mandible), ligamentousconnections of these bones to the skull and to each other (premaxilla to maxilla, ethmoid, and rostrum; maxilla to mandible, palatine, and suspensorium; mandible to suspensorium),and several muscles, notably the expaxials,levator operculi, hypaxials, adductor mandibulae, andlevator arcus palatini (Fig. 8.4).
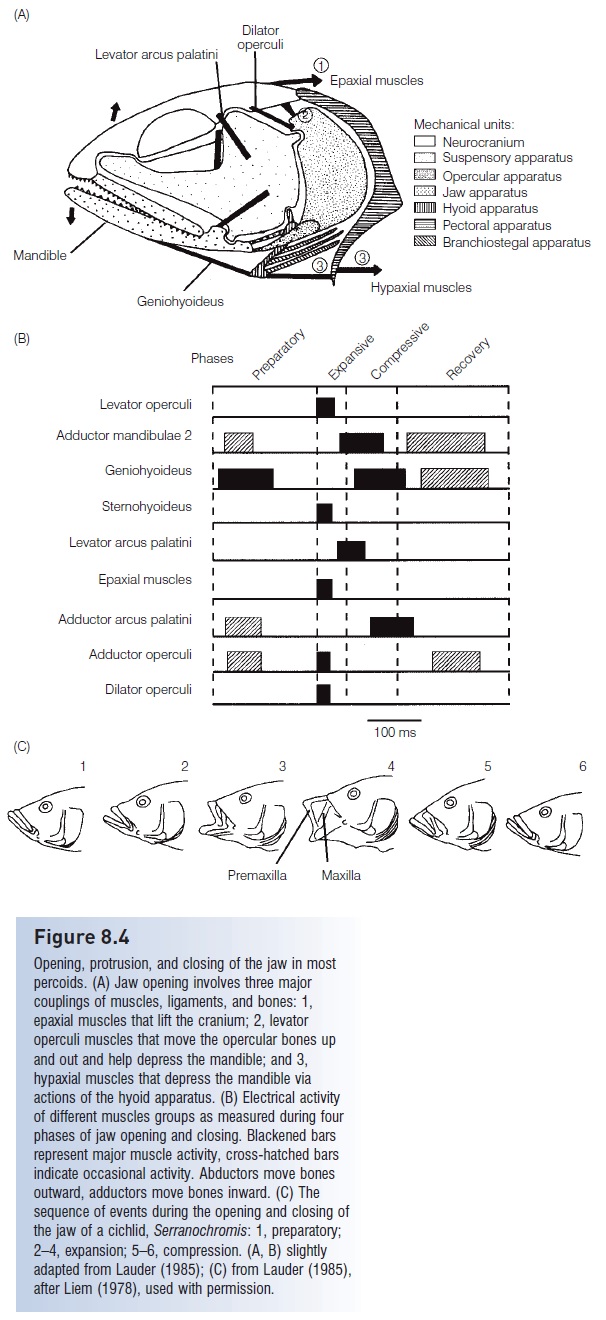
Figure 8.4
Opening, protrusion, and closing of the jaw in mostpercoids. (A) Jaw opening involves three major couplings of muscles, ligaments, and bones: 1,epaxial muscles that lift the cranium; 2, levatoroperculi muscles that move the opercular bones upand out and help depress the mandible; and 3,hypaxial muscles that depress the mandible viaactions of the hyoid apparatus. (B) Electrical activityof different muscles groups as measured during four phases of jaw opening and closing. Blackened bars represent major muscle activity, cross-hatched bars indicate occasional activity. Abductors move bones outward, adductors move bones inward. (C) Thesequence of events during the opening and closing of the jaw of a cichlid, Serranochromis: 1, preparatory;2–4, expansion; 5–6, compression. (A, B) slightlyadapted from Lauder (1985); (C) from Lauder (1985),after Liem (1978), used with permission.
During jaw protrusion, the entire jaw moves forward and slightly up or down. Protrusion in a generalized percomorphoccurs as the cranium is lifted by the epaxialmuscles and the lower jaw is depressed by muscles associatedwith the opercular and hyoid bone series. Movement of the mandible causes the maxillary to pivot forward, thesuspensorium (the hinge joint that suspends the lower jaw from the cranium) contributing to maxillary rotation. The descending process of the premaxilla is connected to the lower edge of the maxilla, so the premaxilla is pushed forward, its ascending process sliding forward and down the rostrum. The jaw is closed through the actions of the adductor mandibulae muscle on the mandible, the levatorarcus palatini on the suspensorium, and the geniohyoideuson the hyoid apparatus. Many variations on this simplified description exist, differing among taxa in terms of twisting of jaw bones, points of attachment and pivot between structures, inclusion of other small bony elements, and actions of muscles and ligaments on particular elements(Motta 1984).
Jaw protrusion creates rapid water flow that carries edible particles, both small and large, into the fish’s mouth. Suction velocity increases from 0 m/s to as much as12 m/s in as little as 0.02–0.03 s (Osse & Muller 1980;Ferry-Graham et al. 2003). Fishes that feed on such different prey as phytoplankton, zooplankton, macroinvertebrates, and other fishes utilize suction to capture prey; the larger the object, the more suction pressure must be produced to capture it. Suction feeding, also known as inertial suction, results from rapid expansion of the buccal (mouth)cavity, which creates negative pressure in the mouth relative to the pressure outside the mouth. Particles in the water mass ahead of the fish are carried into the mouth along with the water. The jaws then close, pushing the water out the gill covers but retaining the prey in the mouth. Gill rakers,jaw teeth, and teeth on various non-marginal jaw bones(palate, vomer, tongue) act to mechanically prevent escapefrom the opercular chamber.
Suction pressures vary during a feeding event in advancedpercomophs, increasing and decreasing four times. Thefour phases of suction feeding are preparation, expansion, compression, and recovery (Lauder 1983a, 1985).
1 During preparation, as the fish approaches its prey, pressure in the buccal cavity increases as a result of inward squeezing of the suspensorium and lifting of the mouth floor.
2 The expansion phase is when maximal suction pressure develops; the mouth is opened to full gapevia lower jaw depression, premaxillary protrusion, and expansion of suspensory, opercular, and mouth floor(hyoid) units. Expansion is the shortest phase during jaw activity, requiring only 5 ms in some anglerfishes. The negative pressures generated during expansion can reach −800 cmH2O (0.7 atm) in the Bluegill Sunfish, approaching the physical limits imposed by fluid mechanics. Such rapidly achieved low pressure causes cavitation, which involves water vapor suddenly coming out of solution and forming small vapor-filled cavities (the bubbles produced behind an accelerating boat propeller result from cavitation) (Lauder 1983a).The popping noise made during feeding by Bluegill may result from the collapse of cavitation bubbles.
3 The compression phase occurs and pressure increases as the mouth is closed by reversing the movements of cranial bones, an activity that requires contraction of a different set of muscles (Fig. 8.4C). The opercular andbranchiostegal valves at the back of the head open up after the jaws close, which allows water but not preyto flow out of the buccal and opercular cavities.
4 Recovery involves a return of bones, muscles, and water pressure to their pre-preparatory positions.
Modifications of this basic plan underscore some rather spectacular derivations that allow specialized feeding activities. In cichlids, the suspensorium and maxilla are mechanically decoupled. Jaw protrusion occurs as a result of movement of the suspensorium, independent of the maxilla. The consequence of this decoupling of suspensorium and maxilla is that the jaw can be protruded via four different pathways: lifting the neurocranium, abducting the suspensorium, lowering the mandible, or swinging the maxilla. Cichlids make use of different combinations of jaw elements and protrusion pathways to feed on different prey types or in different habitats (e.g., Waltzek & Wainwright2003; Hulsey & De Leon 2005). High-speed motion picture analysis of jaw action indicates that some cichlids may use eight different feeding patterns in which they vary their gape, biting force, and amount of jaw protrusion depending on the prey type, location, and behavior. Thecichlid jaw is the closest that fishes have come to a prehensile feeding tool. Cichlids show a diversity of foraging types unequaled in any other fish family. It is likely that the derived trait of a decoupledsuspensorium and the resulting trophic versatility have contributed greatly to their success (Liem 1978; Lauder 1981;Motta 1984; Liem & Wake 1985).
Fishes other than cichlids have reworked the basic elements of jaw protrusion and have evolved dramatic specializations that increase attack velocity or suction. As mentioned, the Pike head, Luciocephalus pulcher, shoots its jaw out, increasing its attack speed from 1.3 to 1.8 m/s.Little suction is generated during a strike. Extreme andrapid jaw protrusion in this species involves modified anterior vertebrae and massive epaxial muscles and tendons that run from the vertebrae to the posterior part of the cranium. Upward flexion of the head, made possible by a highly bendable neck, leads to extreme jaw protrusion. Other predators have converged on analogous neck-bending abilities to increase prey capture efficiency, including a characin and two cyprinids (Lauder & Liem 1981).
In most fishes, suction pressure is produced via expansion of the buccal cavity. A generalized perciform such as the Yellow Perch increases its mouth cavity volume by a factor of six, creating a negative pressure capable of supporting a water column about 15 cm high. The apparent record for volume increase is held by a small (30 cm long),bizarre, elongate midwater fish, Stylophorus chordatus.Stylophorus,among its other oddities, has a tubular mouth and a membranous pouch that stretches dorsally from its mouth to its braincase. During feeding, the fish throws its head back and thrusts its tubular mouth forward. The mouth becomes separated from the braincase by a distance of about 1 cm, the intervening space being filled by the now expanded membranous pouch. Mouth volume increases almost 40-fold, creating pressures three times greater than in the generalized perch. The fish engulfs copepods as water rushes in at a calculated velocity of 3.2 m/s, from as far away as 2 cm (Pietsch 1978).
Another extreme of jaw protrusion occurs in the tropical Sling-jaw Wrasse, Epibulus insidiator (West neat&Wainwright 1989). Sling-jaws protrude their jaws up to65% of their unexpended head length, which twice the extension is found in any other fish (Fig. 8.5). This extreme protrusion is accomplished via a major reworking of many jaw elements. Several bones in the Sling-jaw’s head have unique sizes and shapes, including the quadrate, interopercle, premaxilla, and mandible. Ligaments connecting these
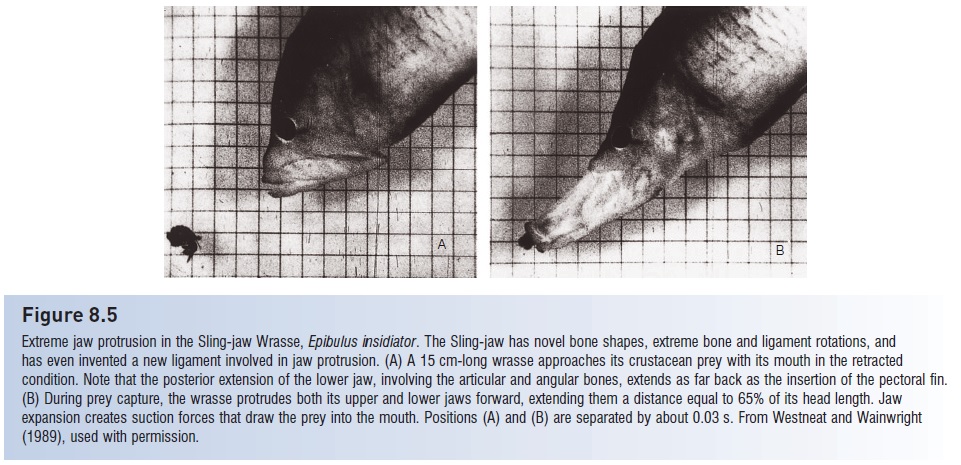
Figure 8.5
Extreme jaw protrusion in the Sling-jaw Wrasse, Epibulus insidiator. The Sling-jaw has novel bone shapes, extreme bone and ligament rotations, and has even invented a new ligament involved in jaw protrusion. (A) A 15 cm-long wrasse approaches its crustacean prey with its mouth in the retracted condition. Note that the posterior extension of the lower jaw, involving the articular and angular bones, extends as far back as the insertion of the pectoral fin.(B) During prey capture, the wrasse protrudes both its upper and lower jaws forward, extending them a distance equal to 65% of its head length. Jaw expansion creates suction forces that draw the prey into the mouth. Positions (A) and (B) are separated by about 0.03 s. From West neat and Wainwright(1989), used with permission.
The modified bones undergo extreme and in some cases unique rotations during jaw protrusion: the lower jaw actually moves forward during protrusion, a departure from the depression movement seen in all other fishes. The Sling-jaw shoots its mouth out at small fishes and crustaceans on coral reef surfaces, suctioning them into its mouth. It achieves a strike velocity of 2.3 m/s, but all of this speed is contributed by the jaw because the fish hovers almost still in the water while attacking prey. Extreme jaw protrusion in Sling-jaws involves the evolution of unique bones and ligaments, but the muscles of the jaw and skull have shapes, functions, and sequences of activity that differ little from generalized perciforms. Novel jaw function is therefore accomplished by drastic modification of some structures and the retention of primitive condition in others. The Sling-jaw exemplifiesa widely made observation about the evolutionary process, that every species represents a mosaic of ancestral and derived traits.
Suction feeding has evolved repeatedly during fish evolution and occurs in many non-teleost’s as well as in primitiveand specialized teleost’s that are unable to protrude their jaws. Elasmobranchs, including skates, rays, and such sharks as nurse and horn sharks, can generate suction forces as strong as −760 mmHg for feeding on buried mollusks or lobsters in reef crevices (Tanaka 1973; Motta & Wilga1999, 2001). Lungfishes and Bowfin among non-teleost’s,and anguillid eels, salmons, pickerels, and triggerfishes amongteleost’s do not protrude their jaws but use inertial suction for feeding; sturgeons have independently evolved jaw protrusion and suction feeding. Suction in the no protrudingspecies is often accomplished by rapid depression of the floor of the mouth. Triggerfishes and other tetraodontiform fishes such as boxfishes can reverse thisflow and forcefully expel water from their mouths (Frazeret al. 1991). Alternate blowing and sucking is used to manipulate food items in the mouth during repositioning for biting. Blowing is also used for uncovering invertebrate prey buried in sand or for manipulating well-defended prey items. A Red Sea triggerfish, Balistes fuscus, feeds on longspine sea urchins. The only spine-free region of the urchinis the oral disk around the mouth. Triggerfishes swim up toan urchin sitting on sand and blow a powerful jet of water at the urchin’s base. The water stream lifts the urchin off the substrate and rolls it over, at which point the triggerfish bites through the now-exposed oral disk, killing the urchin (Fricke 1973). Triggerfishes also use blowing to uncover buried prey such as sand dollars. Blowing involves compression of the mouth via actions of muscles associated with the opercular, mandibular, and hyoid bones (Frazer et al.1991; Turing an& Wainwright 1993).
Related Topics