Chapter: Introduction to Human Nutrition: Minerals and Trace Elements
Iron: Toxicity, Genetic diseases, Requirements, dietary sources, Micronutrient interactions
Iron
Iron is a relatively abundant element in the universe.
It is found in the sun and many types of stars in considerable quantity. The core of the Earth is thought to be largely composed of iron and it makes up 4.7% of the Earth’s crust. The most common ore is hematite, which is frequently seen as black sands along beaches and streams. Taconite is becoming increasingly important as a commercial ore. Because iron is easy to obtain, its discovery is lost in the history of man, many thousands of years ago. The early Greeks were aware of the health-giving properties of iron. Iron has been used for centuries as a health tonic. It is therefore paradoxical that although the need for iron was discovered long ago and although it is the most common and cheapest of all metals, iron deficiency is probably the most frequent deficiency disorder in the world and the main remaining nutritional deficiency in Europe. Iron can exist in oxidation states ranging from −2 to +6. In biological systems, these oxidation states occur primarily as the ferrous (Fe2+) and ferric (Fe3+) forms and these are interchangeable.
Toxicity
The very effective regulation of iron absorption pre-vents overload of the tissues by iron from a normal diet, except in individuals with genetic defects, as in idiopathic hemochromatosis . Excess iron via overuse of iron supplements could pose a possible health risk. The mechanism of cellular and tissue injury resulting from excess iron is not fully under-stood. Liabilities may include increased risks for bac-terial infection, neoplasia, arthropathy, cardiomyopa-thy, and endocrine dysfunctions. However, there is still much debate as to the strength of evidence to support a relationship between dietary iron intake and cancer or cardiovascular disease.
Gastrointestinal distress does not occur from consuming a diet containing naturally occurring or fortified iron. Individuals taking iron at high levels (>45 mg/day) may encounter gastrointes-tinal side-effects (constipation, nausea, vomiting, and diarrhea), especially when taken on an empty stomach. Based largely on the data on gastrointestinal effects following supplemental elemental iron intake in apparently healthy adults, the US Food and Nutri-tion Board established a tolerable UL of iron of 45 mg/day.
Genetic diseases
Primary idiopathic hemochromatosis is a hereditary disorder of iron metabolism characterized by an abnormally high iron absorption owing to a failure of the iron absorption control mechanism at the intes-tinal level. High deposits of iron in the liver and the heart can lead to cirrhosis, hepatocellular cancer, con-gestive heart failure, and eventual death. Sufferers of this disorder can develop iron overload through con-sumption of a normal diet, but would be at much higher risk if consuming iron-fortified foods. Thus, early detection of the disease via genetic screening followed by regular blood removal has proven to be a successful treatment.
Assessing status
Several different laboratory methods must be used in combination to diagnose iron deficiency anemia cor-rectly. The most commonly used methods to assess iron status include:
● serum ferritin
● transferrin saturation
● erythrocyte protoporphyrin
● mean corpuscular volume
● serum transferrin receptor
● hemoglobin or packed cell volume.
Iron deficiency anemia is usually defined as a hemo-globin level below the cut-off value for age and sex plus at least two other abnormal iron status measure-ments. The most commonly used are probably low serum ferritin, high protoporphyrin, and, more recently, high serum transferrin receptor.
Requirements and dietary sources
Daily (absorbed or physiological) iron requirements are calculated from the amount of dietary iron neces-sary to cover basal iron losses, menstrual losses, and growth needs. They vary according to age and sex and, in relation to body weight, they are highest for the young infant. An adult man has obligatory iron losses of around 1 mg of iron/day, largely from the gastrointestinal tract (exfoliation of epithelial cells and secretions), skin, and urinary tract. Thus, to remain replete with regard to iron, an average adult man needs to absorb only 1 mg of iron from the diet on a daily basis. Similar obligatory iron losses for women amount to around 0.8 mg/day. However, adult women experience additional iron loss owing to menstruation, which raises the median daily iron requirement for absorption to 1.4 mg (this covers 90% of menstruating women; 10% will require daily absorption of at least 2.4 mg iron to compensate for their very high menstrual losses). Pregnancy creates an additional demand for iron, especially during the second and third trimesters, leading to daily require-ments of 4–6 mg. Growing children and adolescents require 0.5 mg iron/day in excess of body losses to support growth. Physiological iron needs can be translated into dietary requirements by taking into account the efficiency at which iron is absorbed from the diet (typically around 10%). Current RDAs for iron (recommended by the US Food and Nutrition Board in 2001) are infants 0.27 mg (first 6 months; this is an adequate intake value), 11 mg (7–12 months), children 7 and 10 mg (1–3 and 4–8 years, respectively), teenage boys 8 and 11 mg (9–13 and 14–18 years, respectively), adult men 8 mg (19 years and older), teenage girls 8 and 15 mg (9–13 and 14–18 years, respectively), adult women 18 and 8 mg (19–50 years and 51 years and older, respectively), pregnant women 27 mg and lactating women 10 and 9 mg (younger than 18 years and 19–50 years, respectively).
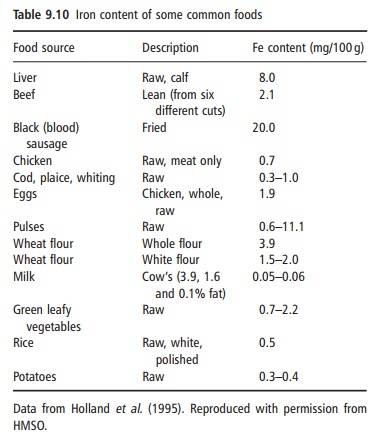
Iron is widely distributed in meat, eggs, vegetables, and cereals, but the concentrations in milk, fruit, and vegetables are low (Table 9.10). The iron content per se of individual foods has little meaning as iron absorption varies considerably. There are two types of food iron: nonheme iron, which is present in both plant foods and animal tissues, and heme iron, coming from the hemoglobin and myoglobin in animal prod-ucts. Heme iron represents 30–70% of the total iron in lean meat and is always well absorbed. Nonheme iron from meat and vegetable foods enters a common nonheme iron pool in the gastric juice, from which the amount of iron absorbed depends to a large extent on the presence of enhancing and inhibiting sub stances in the meal and on the iron status of the individual.
Micronutrient interactions
The fact that serum copper has been found to be low in some cases of iron deficiency anemia suggests that iron status has an effect on copper metabolism. Copper deficiency impinges on iron metabolism, causing an anemia that does not respond to iron supplementation. Interactions between iron and copper seem to be owing to impaired utilization of one in the absence of the other. As mentioned above, calcium can inhibit iron absorption under certain cir-cumstances. In aqueous solutions iron impairs zinc absorption, but this interaction does not take place when iron is added to an animal protein meal, indi-cating different uptake mechanisms for solutions and solid foods.
Related Topics