Chapter: Modern Analytical Chemistry: Spectroscopic Methods of Analysis
Instrument Designs for Infrared Absorption - Ultraviolet-Visible and Infrared Spectrophotometry
Instrument Designs
for Infrared Absorption
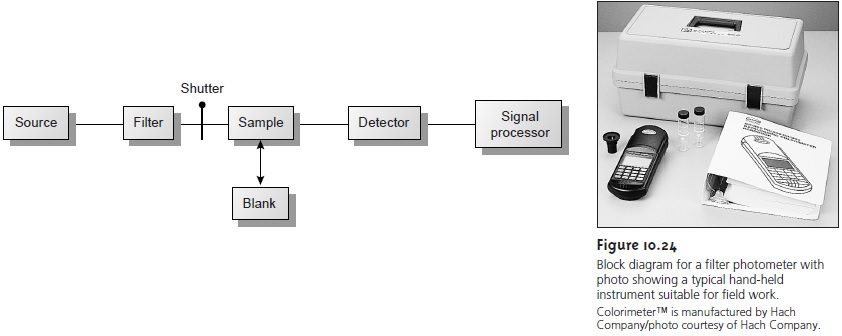
The simplest instrument for IR ab- sorption spectroscopy is a filter photometer similar to that shown in Figure 10.24 for UV/Vis absorption. These
instruments have the
advantage of portability and typically are used
as dedicated analyzers for gases such
as HCN and
CO.
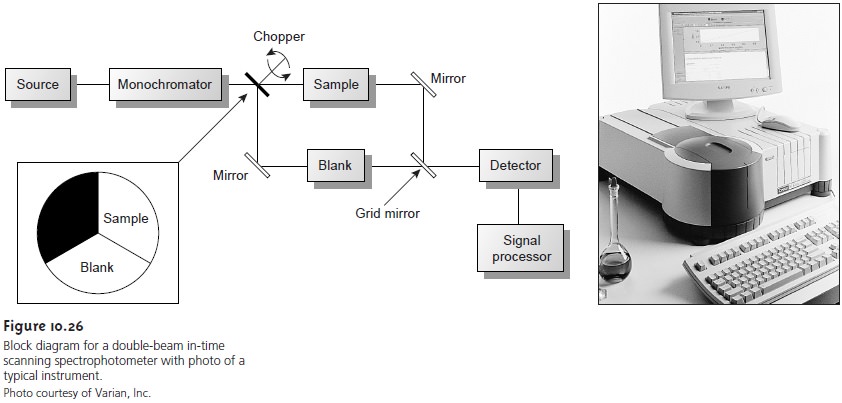
Infrared instruments using
a monochromator for
wavelength selection are
con- structed using double-beam optics similar to that shown in Figure 10.26. Double- beam optics are preferred over single-beam optics
because the sources
and detectors for infrared radiation are less
stable than that
for UV/Vis radiation. In addition, it is
easier to correct for the
absorption of infrared radiation by atmospheric CO2 and
H2O vapor when using double-beam optics. Resolutions of 1–3 cm–1 are typical
for most instruments.

In a Fourier transform, infrared spectrometer, or FT–IR, the
monochromator is replaced with an interferometer (see Figure 10.13). Because an
FT–IR includes only a single optical path, it is necessary to collect a separate spectrum
to compen- sate for the absorbance of atmospheric CO2 and H2O vapor. This is done by collect-
ing a background spectrum without
the sample and storing the result in the instru- ment’s computer memory. The background spectrum
is removed from the sample’s spectrum by ratioing the two signals.
In comparison to other IR instruments, an FT–IR provides for rapid data
acquisition, allowing an enhancement in signal-to- noise ratio through signal
averaging.
Infrared spectroscopy is routinely used for the analysis of samples in the gas, liquid, and solid states.
Sample cells are made from materials, such as NaCl and
KBr, that are transparent to infrared radiation. Gases are analyzed
using a cell with a pathlength of approximately 10 cm. Longer pathlengths are obtained by using mir- rors
to pass the
beam of radiation through the sample
several times.
Liquid samples are analyzed in one of two ways. For nonvolatile liquids a suit- able sample can be prepared by placing a drop of the liquid
between two NaCl plates, forming a thin film that typically is less than 0.01 mm thick. Volatile
liquids must be placed
in a sealed cell to prevent their
evaporation.
The analysis of solution samples
is limited by the solvent’s IR-absorbing prop-
erties, with CCl4, CS2, and CHCl3 being
the most common
solvents. Solutions are placed in cells containing
two NaCl windows separated
by a Teflon spacer. By changing the Teflon spacer,
pathlengths from 0.015
to 1.0 mm can be obtained.
Sealed cells with fixed or variable pathlengths also are available.
The analysis of aqueous solutions
is complicated by the solubility of the NaCl cell
window in water.
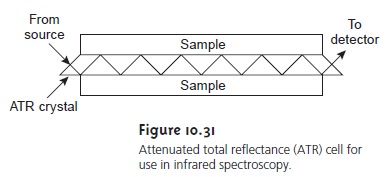
One approach to obtaining infrared
spectra on aqueous
solu- tions is to use attenuated total reflectance (ATR) instead oftransmission.
Figure 10.31 shows a diagram of
a typical ATR sampler, consisting of an IR-transparent crystal of high- refractive index, such as ZnSe, surrounded by a sample
of lower-refractive index.
Radiation from the source enters
the ATR crystal, where
it undergoes a series of total internal
reflec- tions before exiting
the crystal. During
each reflection, the ra
diation penetrates into the sample
to a depth of a few microns.
The result is a selec- tive attenuation of the radiation
at those wavelengths at which the sample absorbs.
ATR spectra are similar, but not identical, to those obtained
by measuring the transmission
of radiation.
Transparent solid samples
can be analyzed directly by placing them in the IR
beam. Most solid samples, however,
are opaque and must be dispersed in a more transparent medium before recording a traditional transmission spectrum. If a suit-
able solvent is available, then the solid can be analyzed by preparing a solution and analyzing as described earlier.
When a suitable solvent is not available, solid samples may be analyzed by preparing a mull of the finely powdered sample with a suitable
oil. Alternatively, the powdered sample
can be mixed with KBr and pressed
into an optically transparent pellet.
Solid samples also can be analyzed by means of reflectance. The ATR sampler (see Figure 10.31) described for the analysis of aqueous solutions can be used
for the analysis of solid samples, provided that the
solid can be brought into
contact with the ATR
crystal. Examples of solids that
have been analyzed by ATR include polymers, fibers, fabrics, powders, and biological tissue samples.
Another re- flectance method
is diffuse reflectance, in which radiation is reflected from a rough surface, such as a powder. Powdered
samples are mixed
with a nonabsorbing mate-
rial, such as powdered KBr,
and the reflected light is collected and analyzed. As with
ATR, the resulting spectrum is similar to that obtained
by conventional transmission methods.
Related Topics