Chapter: Biochemistry: Amino Acids and Peptides
Individual Amino Acids:Their Structures and Properties
Individual Amino Acids:Their
Structures and Properties
Why are amino acid side chains so important?
The R
groups, and thus the individual amino acids, are classified according to
several criteria, two of which are particularly important. The first of these
is the polar or nonpolar nature of the side chain. The second depends on the
presence of an acidic or basic group in the side chain. Other useful criteria
include the presence of functional groups other than acidic or basic ones in
the side chains and the nature of those groups.
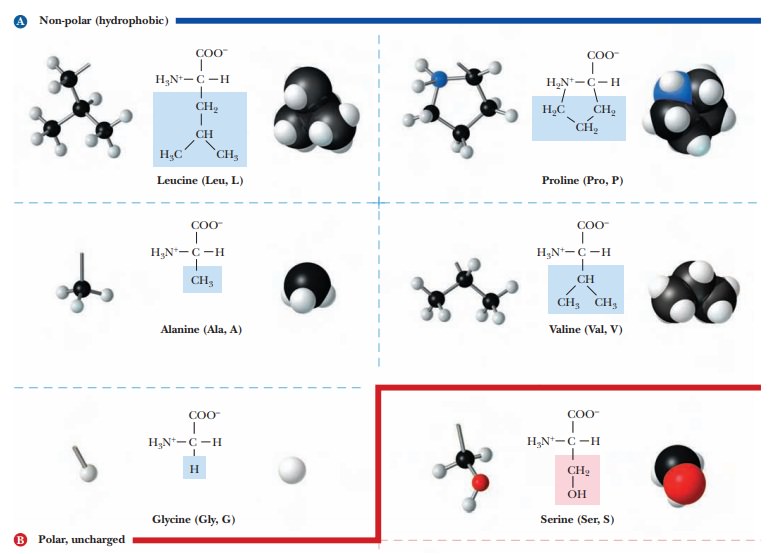

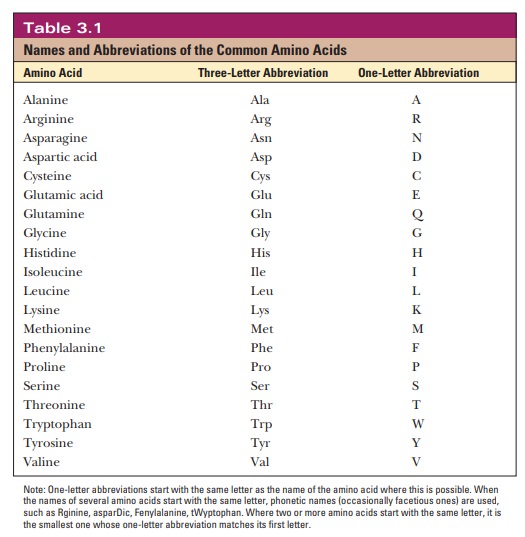
As mentioned, the side chain of the simplest amino acid, glycine, is a hydrogen atom, and in this case alone two hydrogen atoms are bonded to the α-carbon. In all other amino acids, the side chain is larger and more complex (Figure 3.3). Side-chain carbon atoms are designated with letters of the Greek alphabet, counting from the α-carbon. These carbon atoms are, in turn, the β-, γ-, δ-, and ε-carbons (see lysine in Figure 3.3); a terminal carbon atom is referred to as the v-carbon, from the name of the last letter of the Greek alpha-bet. We frequently refer to amino acids by three-letter or one-letter abbreviations of their names, with the one-letter designations becoming much more prevalent these days; Table 3.1 lists these abbreviations.
Which amino acids have nonpolar side chains? (Group 1)
One
group of amino acids has nonpolar side chains. This group consists of glycine,
alanine, valine, leucine, isoleucine, proline, phenylalanine, tryptophan, and
methionine. In several members of this group-namely alanine, valine, leucine,
and isoleucine-each side chain is an aliphatic hydrocarbon group. (In organic
chemistry, the term aliphatic refers
to the absence of a benzene ring or related structure.) Proline has an
aliphatic cyclic structure, and the nitrogen is bonded to two carbon atoms. In
the terminology of organic chemistry, the amino group of proline is a secondary
amine, and proline is often called an imino
acid. In contrast, the amino groups of all the other common amino acidsare
primary amines. In phenylalanine, the hydrocarbon group is aromatic (it
contains a cyclic group similar to a benzene ring) rather than aliphatic. In
tryptophan, the side chain contains an indole ring, which is also aromatic. In
methionine, the side chain contains a sulfur atom in addition to aliphatic
hydrocarbon groupings. (See Figure 3.3.)
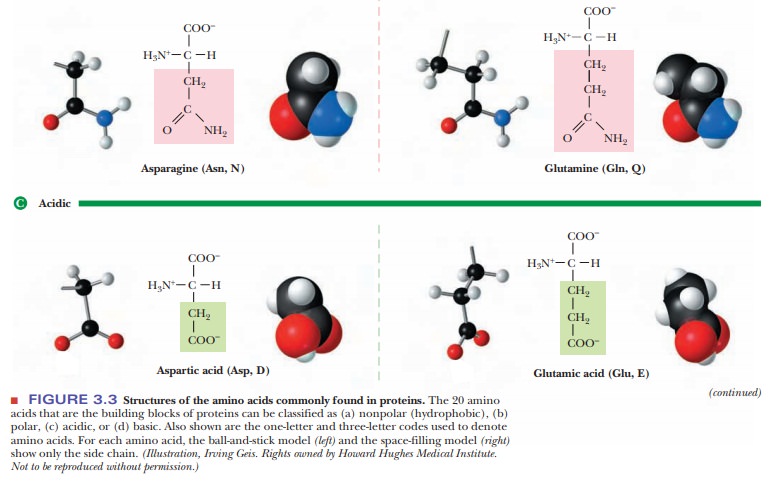
Which amino acids have electrically neutral polar side chains? (Group 2)
Another group of amino acids has polar side chains that are electrically neutral (uncharged) at neutral pH. This group includes serine, threonine, tyrosine, cysteine, glutamine, and asparagine. Glycine is sometimes included here for convenience because it lacks a nonpolar side chain.
In
serine and threonine, the polar group is a hydroxyl (-OH) bonded to aliphatic
hydrocarbon groups. The hydroxyl group in tyrosine is bonded to an aromatic
hydrocarbon group, which eventually loses a proton at higher pH. (The hydroxyl
group in tyrosine is a phenol, which is a stronger acid than an aliphatic
alcohol. As a result, the side chain of tyrosine can lose a proton in a
titration, whereas those of serine and threonine would require such a high pH
that pKa values
are not normally listed for these side chains.) In cysteine, the polar side
chain consists of a thiol group (-SH), which can react with other cysteine
thiol groups to form disulfide (-S-S-) bridges in proteins in an oxidation
reaction. The thiol group can also lose a proton. The amino acids glutamine and
asparagine have amide groups, which are derived from carboxyl groups, in their
side chains. Amide bonds do not ionize in the range of pH usually encountered
in biochemistry. Glutamine and asparagine can be considered derivatives of the
Group 3 amino acids, glutamic acid and aspartic acid, respectively; those two
amino acids have carboxyl groups in their side chains.
Which amino acids have carboxyl groups in their side chains? (Group 3)
Two
amino acids, glutamic acid and aspartic acid, have carboxyl groups in their
side chains in addition to the one present in all amino acids. A carboxyl group
can lose a proton, forming the corresponding carboxylate anion-glutamate and
aspartate, respectively, in the case of these two amino acids. Because of the
presence of the carboxylate, the side chain of each of these two amino acids is
negatively charged at neutral pH.
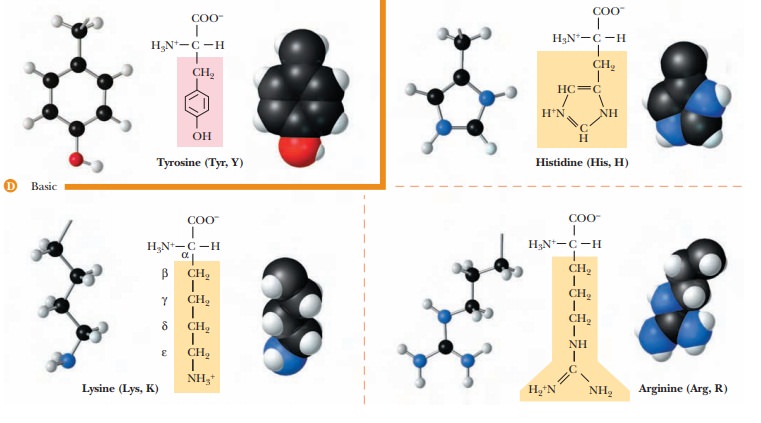
Which amino acids have basic side chains? (Group 4)
Three
amino acids-histidine, lysine, and arginine-have basic side chains, and the
side chain in all three is positively charged at or near neutral pH. In lysine,
the side-chain amino group is attached to an aliphatic hydrocarbon tail. In
arginine, the side-chain basic group, the guanidino group, is more complex in
structure than the amino group, but it is also bonded to an aliphatic
hydrocarbon tail. In free histidine, the pKa of the
side-chain imidazole group is 6.0, which is not far from physiological pH. The
pKa values
for amino acids depend on the environment and can change significantly within
the confines of a protein. Histidine can be found in the protonated or
unprotonated forms in proteins, and the properties of many proteins depend on
whether individual histidine residues are or are not charged.
Uncommon Amino Acids
Which amino acids are found less commonly in proteins?
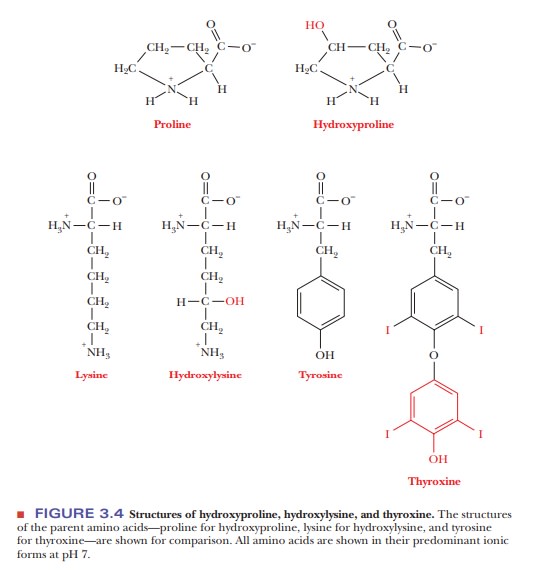
Many other amino acids, in addition to the ones listed here, are known to exist. They occur in some, but by no means all, proteins. Figure 3.4 shows some examples of the many possibilities. They are derived from the common amino acids and are produced by modification of the parent amino acid after the protein is synthesized by the organism in a process called posttranslational modification. Hydroxyproline and hydroxylysine differ from the parent amino acids in that they have hydroxyl groups on their side chains; they are found only in a few connective-tissue proteins, such as collagen. Thyroxine differs from tyrosine in that it has an extra iodine-containing aromatic group on the side chain; it is produced only in the thyroid gland, formed by posttranslational modification of tyrosine residues in the protein thyroglobulin. Thyroxine is then released as a hormone by proteolysis of thyroglobulin.
Summary
Amino acids are classified according to two
major criteria: the polarity of the side chains and the presence of an acidic
or basic group in the side chain.
Four
groups of amino acids are found in proteins: first, those with non-polar side
chains; second, those with electrically neutral polar side chains; third, those
with carboxyl groups in their side chains; fourth, those with basic side
chains.
Related Topics