Chapter: Medical Immunology: The Humoral Immune Response and Its Induction by Active Immunization
Immunization
IMMUNIZATION
A. Historical Background
The concept of active immunization as a way to prevent infectious diseases is about two centuries old, if we consider the introduction of cowpox vaccination by Jenner in 1796 as
Jenner observed that milkmaids that had contracted cowpox were pro-tected from smallpox and developed an immunization procedure based on the intradermal scarification of material from cowpox lesions. Empirically, he had discovered the principle of vaccine with live, attenuated microbes, which was later picked up by Louis Pasteur when he developed several of his vaccines. As infectious agents became better characterized, new vaccines were developed, some with inactivated organisms, others with microbial components, others still with attenuated infectious agents. Mass vaccination has had some remarkable successes, such as the eradication of smallpox and the significant declines in some of the most common or most serious infectious diseases of childhood, such as measles and polio. At the present time we are witnessing a new burst of progress in vaccine devel-opment, reflecting the application of molecular genetics techniques to the rational devel-opment of immunizing agents.
B. Types of Vaccines
A wide variety of immunizing agents has been developed. The following are some exam-ples of the types of immunizing agents that are used for immunoprophylaxis in humans.
1. Killed Vaccines
Killed vaccines are generally safe, but they are not as effective as attenuated vaccines.
1. Killed bacteria include the traditional pertussis vaccine prepared with killed Bor-detella pertussis, the etiological agent of whooping cough, the typhoid vaccineprepared with acetone-inactivatedSalmonella typhi, and the cholera vaccine, prepared with killed Vibrio cholerae. The killed B. pertussis vaccine was re-ported to cause neurological reactions similar to autoallergic encephalitis, par-ticularly in children with a history of neonatal or postnatal seizures. However, these reactions are extremely rare and were mostly observed in Great Britain.
2. Inactivated viruses include the influenza vaccine, the hepatitis A vaccine, and Salk’s polio vaccine. The inactivated poliovaccine contains a mixture of the three known types of poliovirus, after inactivation with formalin. This vaccine has been as successful in the eradication of poliomyelitis as Sabin’s attenuated oral vaccine. Its main advantage is safety, but it is not as effective or amenable to mass immunizations as the oral vaccine . However, safety con-cerns have resulted in its wider use in countries where poliomyelitis has been vir-tually eradicated, and there is greater risk of contracting polio from the attenu-ated vaccine than from a wild virus strain.
2. Component Vaccines
Component vaccines are even safer than killed vaccines, but their efficacy can be prob-lematic.
Bacterial Polysaccharides.Bacterial polysaccharides, such as those used for Streptococcus pneumoniae, Neisseria meningitidis, and Haemophilus influenzae type b,and for a typhoid fever vaccine made of the Vi capsular polysaccharide. Because of their T-independent nature, polysaccharide vaccines are not very potent (especially in young children) and do not elicit long-lasting memory .
Inactivated Toxins.Inactivated toxins (toxoids), such as tetanus and diphtheriatoxoids, which are basically formalin-inactivated toxins, have lost their active site but maintained their immunogenic determinants. Toxoids are strongly immunogenic proteins and induce antibodies able to neutralize the toxins. The response to toxoids is associated with long-lasting memory.
Recombinant Bacterial Antigens.Recently, a recombinantRickettsia rickettsiiantigen produced in E. coli has been proposed as a candidate vaccine for Rocky Mountain spotted fever. Recombinant toxoids (e.g., recombinant pertussis toxin) have also been in-troduced in human vaccines. These differ from classical toxoids in that they are produced by genetically engineered organisms to which an altered gene coding for an inactive toxin (toxoid) has been introduced.
Mixed Component Vaccines.The interest in developing safer vaccines forwhooping cough led to the introduction of acellular vaccines. These are constituted by a mixture of inactivated pertussis toxin or recombinant pertussis toxin (nontoxic due to the deletion of critical domains), a major determinant of the clinical disease, and one or several adhesion factors, which mediate attachment to mucosal epithelial cells. These vaccines have replaced the old vaccine prepared with killed Bordetella pertussis.
Conjugate Vaccines.Most polysaccharide vaccines have shown poor immuno-genicity, particularly in infants. This lack of effectiveness is a consequence of the fact that polysaccharides tend to induce T-independent responses with little immunological mem- ory. This problem appears to be eliminated if the polysaccharide is conjugated to an im-munogenic protein, very much like a hapten-carrier conjugate.
The first conjugate vaccines to be developed involved the polyribositolribophosphate (PRP) of Haemophilus influenzae type b (Hib). Four conjugate vaccines have been suc-cessfully tested, the first three being currently approved by FDA:
1. PRP-OMPC, in which the carrier is an outer membrane protein complex of Neis-seria meningitidis
2. Hib-OC, in which the carrier (OC) is a nontoxic mutant of diphtheria toxin
3. PRP-T, in which the carrier is diphtheria toxoid
The introduction of these vaccines was followed by a 95% decrease in the incidence of H.influenzae type b infections affecting children younger than 5 years of age.
A conjugate vaccine prepared with the capsular polysaccharides of the seven most common types of Streptococcus pneumoniae has received FDA approval for use in the pe-diatric population, and a conjugate vaccine for Neisseria meningitidis is currently being evaluated.
Viral Component Vaccines.Viral component vaccines are based on the immuno-genicity of isolated viral constituents. The best example is the hepatitis B vaccine, produced by recombinant yeast cells. The gene coding for the hepatitis B surface antigen (HBsAg) was isolated from the hepatitis B virus and inserted into a vector, flanked by promoter and terminator sequences. That vector was used to transform yeast cells, from which HBsAg was purified. All the available hepatitis B vaccines are obtained by this procedure.
Some of the proposed HIV vaccines are component vaccines, constituted by enve-lope glycoproteins (gp160 or its fragment, gp120) or peptides derived from these glyco-proteins, produced in genetically engineered E. coli, insect cells, and mammalian cell lines. These vaccines have not been proven to induce protective immunity .
Synthetic Peptide Vaccines.The use of synthetic peptides for vaccination has theadvantages of easy manufacture and safety. The goal is to synthesize the peptide sequences corresponding to known epitopes recognized by neutralizing antibodies and use them as vaccines. This theoretically appealing concept meets with two basic problems. First, it is highly questionable that a synthetic oligopeptide has the same tertiary configuration as the epitope expressed by the native antigen and that protective antibodies can be elicited in this manner. However, if the objective is to induce cell-mediated immunity, this may not be an insurmountable obstacle. Second, small synthetic peptides are poorly immunogenic. The use of peptide-protein (e.g., tetanus toxoid) conjugates can minimize this problem.
The most promising work with synthetic peptide vaccines has been carried out with Plasmodium peptides. In a murine malaria model, immunization with a tetanustoxoid–Plasmodium berghei peptide conjugate resulted in rates of protection ranging from 75 to 87%, identical to those observed with a killed vaccine made of the whole parasite. In humans, efforts have been concentrated on the development of a vaccine against Plasmod-ium falciparum. A multirepeat region (approximately 40 repeats of the sequence Asn-Ala-Asn-Pro) of the circumsporozoite protein was identified as the immunodominant B-cell epitope and used as a model for a peptide-based vaccine. However, the rate of protection obtained in the first trials with this vaccine was too low (two out of nine subjects immu-nized were protected), probably because of differences in tertiary structure between the synthetic peptide and the Plasmodium epitope.
DNA Vaccines.The observation that intramuscular injection of nonreplicating plas-mid DNA encoding the hemagglutinin (HA) or nucleoprotein (NP) of influenza virus elicited humoral and cellular protective reactions attracted enormous interest from the scientific com-munity. The recombinant DNA is taken up and expressed by APCs at the site of injection and is presented to T-helper cells in a way that both humoral and cell-mediated immune responses are elicited. The safety and easy storage of candidate DNA vaccines are extremely appealing and several different trials are ongoing. However, the initial impression from human trials is that DNA vaccines are far less potent in humans than they appear to be in experimental ani-mals. At this point is too early to pass judgment on the practical value of DNA vaccines.
C. Attenuated Vaccines
Attenuated vaccines are generally very efficient but in rare cases can cause the very disease they are designed to prevent, particularly in immunocompromised individuals. Most an-tiviral vaccines are made of viral strains attenuated in the laboratory, including the classi-cal smallpox vaccine, the oral polio vaccine (a mixture of attenuated strains of the three known types of poliovirus), the mumps-rubella-measles vaccine, and the varicella-zoster vaccine, recently approved by FDA. Attenuated viral vaccines tend to be very potent, prob-ably because of the infective nature of the immunizing agent. In the case of poliovaccines, the attenuated virus can be transmitted by the fecal-oral route to nonimmunized individu-als, thus increasing the proportion of immunized individuals in any given population. An-other advantage of attenuated vaccines administered topically (by mouth or aerosol) is the simplicity of the immunization procedure. Thus, topically administered vaccines are ide-ally suited to mass immunization.
The bacillus of Calmette-Guérin (BCG), an attenuated strain of Mycobacterium bo-vis, has been used for decades as a vaccine for tuberculosis. Unfortunately the rates of pro-tection obtained with this vaccine are rather variable, from 80% to 0%. The trials conducted in the United States were particularly disappointing and resulted in the lack of interest in BCG as an immunoprophylactic agent.
The application of molecular genetics techniques has resulted in the development of attenuated bacteria, which are finding applications in immunoprophylaxis. An attenuated strain of Salmonella typhi that grows poorly and is virtually nonpathogenic but induces pro-tective immunity in 90% of the individuals is the recommended vaccine for typhoid fever. Mutant Bordetella strains that code for an immunogenic toxin lacking their binding sites (thus devoid of pathogenic effects) are being field-tested as whooping cough vaccines.
4. Recombinant Organisms
Recombinant technology has also been used to delete the genes coding for virulence fac-tors from bacteria, creating genetically attenuated strains, as well as to add genetic infor-mation to attenuated viruses or attenuated bacteria, creating recombinant organisms to be used as immunizing agents. Recombinant vaccinia viruses, in which the genetic informa-tion coding for relevant antigens of unrelated viruses has been added to the vaccinia virus genome, have been developed and used successfully. Since the genome of vaccinia virus is rather large, multiple recombinant constructs carrying simultaneously the genes for the HBsAg, glycoprotein D for herpes virus, and influenza virus hemagglutinin, have been suc-cessfully generated. The potential value of these constructs is obvious, since they could in-duce protection against multiple diseases after a single immunization. In less ambitious an-imal trials, a recombinant vaccinia virus, carrying a retroviral env gene, protected mice against Friend leukemia virus. Another type of recombinant vaccinia virus expressing an immunodominant region of streptococcal M protein has been shown to reduce streptococ-cal colonization in mice after intranasal immunization.
Experimental vaccines for acquired immunodeficiency syndrome (AIDS) were de-veloped by incorporating parts of the env gene of human immunodeficiency virus (HIV) into the vaccinia virus genome. Initial data suggested that recombinant HIV coding for the gp 120 vaccines of this type were more efficient in inducing cell-mediated immunity than component vaccines containing the same viral glycoprotein. A major concern, however, is the possibility of causing severe infections with this virus if it administered to individuals whose immune system is debilitated.
Recently there has been interest in generating recombinant BCG organisms as po-tentially more effective vaccines for tuberculosis. One of the earlier concepts was to add to the BCG genome the genes coding for IL-2, IL-4, IL-6, GM-CSF, and IFN-γ . This recom-binant BCG was shown to induce strong protective immunity in an animal model. If this approach could be safely translated to the human vaccine, it would constitute a major med-ical breakthrough.
C. Recommended Immunizations
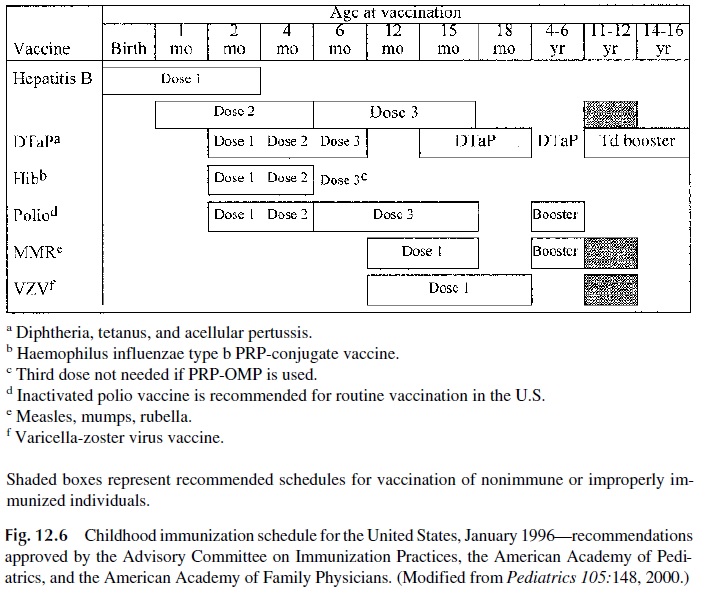
At the present time, a wide variety of vaccines are available for protection of the general population or of individuals at risk for a specific disease due to their occupation or to other factors. Fig. 12.6 summarizes the recommended schedule for active immunization of nor-mal infants and children. Additional information concerning recommended immunizations for adults, travelers, special professions, etc., can be obtained in a variety of specialized publications, including the Report of the Committee on Infectious Diseases, published an-nually by the American Academy of Pediatrics, the booklet Health Information of Interna-tional Travel, also published annually by the U.S. Public Health Service, and the Morbid-ity and Mortality Weekly Report published by the Centers for Disease Control in Atlanta.
D. Vaccines as Immunotherapeutic Agents
The use of vaccines to stimulate the immune system as therapy for chronic or latent infec-tions is receiving considerable interest. Four areas of application have emerged:
1. Herpesvirus infections, in which vaccination seems to reduce the rate of recur-rence
2. Leprosy, in which administration of BCG seems to potentiate the effects of chemotherapy
3. Tuberculosis, in which a new vaccine made of killed Mycobacterium vaccae seems also to potentiate the effects of antituberculosis drugs, even in patients re-sistant to therapy
4. HIV infection, in which vaccination with killed or recombinant HIV may alter the TH1/TH2 balance in favor of TH1, more effective mediators of anti-HIV re-sponses. This approach is being actively pursued as part of immune reconstitu-tion regimens for HIV-positive patients .
Related Topics