Chapter: Modern Pharmacology with Clinical Applications: Diuretic Drugs
Hypokalemia and Potassium-sparing Diuretics
Hypokalemia and Potassium-sparing
Diuretics
Hypokalemia
The chronic use of some
diuretics may require the oral administration of potassium supplements or
potassium-sparing diuretics that reduce urinary K+ excretion. This
is true especially for patients with congestive heart fail-ure and cirrhosis,
who are particularly sensitive to K+ loss. The presence or absence
of clinical symptoms of hypokalemia is quite closely related to serum K+ con-centrations,
and even small changes in extracellular K+ can have marked effects.
Most patients begin to show symptoms when serum K+ levels fall below
2.5 mEq/L (from a normal value of approximately 5 mEq/L).
Neurological symptoms include
drowsiness, irritabil-ity, confusion, loss of sensation, dizziness, and coma.
Other important symptoms of hypokalemia are muscu-lar weakness, cardiac
arrhythmias, tetany, respiratory ar-rest, and increased sensitivity of the
myocardium to dig-italislike drugs.
Treatment
Hypokalemia can be treated by
supplying additional K+ through the diet, drug treatment, or both.
Replacement should be gradual, with frequent evalua-tion of both serum K+ concentrations
and cardiac activ-ity (electrocardiographic monitoring). K+ supplements
can be administered in several forms. KCl is generally preferred over other
forms such as bicarbonate, citrate, or gluconate, since most patients exhibit
concurrent metabolic alkalosis. KCl corrects both the hypokalemia and the
alkalosis. When hypokalemia is not attended by metabolic alkalosis, other forms
of K+ supplementation may be preferred. Since KCl solutions have a
rather bit-ter and unpleasant taste, this salt was formerly given as an
enteric-coated tablet. However, the rapid release of KCl from the tablet after
it entered the small intestine was responsible for a severe local ulceration,
hemor-rhage, and stenosis, especially when there was a delay in gut transit
time; therefore, the enteric-coated tablets have been withdrawn.
Sugar-coated products have
been marketed that contain KCl in a wax matrix (Slow-K+ and Kaon-Cl)
and are purportedly slow- and controlled-release prepara-tions. Available
evidence indicates that these slow-release forms of KCl are occasionally
capable of causing local tissue damage and therefore probably should be used
with caution for K+ supplementation. Solutions of potassium
gluconate, like the tablets, also have been as-sociated with intestinal
ulceration. Microencapsulated KCl preparations (Micro-K, K-Dur) that are neither en-teric coated nor contained
within a wax matrix appear to be superior to the wax matrix formulation.
Consumption of potassium-rich
foods is the easiest and most generally advised means of counteracting a K+
deficit. Table 21.3 lists foods that are suitable for K+ supplementation.
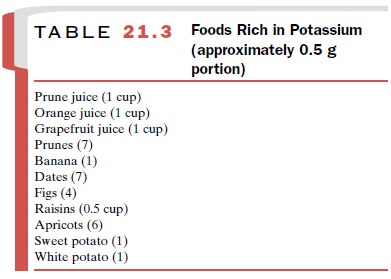
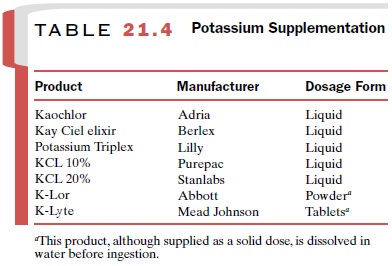
In general, a normal diet plus about 40 mEq per day of K+ is adequate to prevent hypokalemia. If K+ -rich foods prove inadequate in replacing large quantities of the electrolyte or if the increased caloric intake that is part of the dietary supplementation is not desirable, oral liquid therapy is the formulation of choice. A listing of these solutions is given in Table 21.4. Although pa-tients may find many of these products unpalatable, their further dilution with water or fruit juice can be helpful. Finally, the addition of a K+ -sparing diuretic to the therapeutic regimen may prove useful.
The three principal
potassium-sparing diuretic agents produce similar effects on urinary
electrolyte composition. Through actions in the distal convoluted tubule and
collecting duct, they cause mild natriuresis and a decrease in K+ and
H+ excretion. Despite their similarities, these agents actually
constitute two groups with respect to their mechanisms of action.
Aldosterone Antagonists: Spironolactone
The mechanism by which NA+
is reabsorbed in coupled exchange with H+ and K+ in the
collecting duct has been discussed previously; that is, NA+ -driven K+
secre-tion is partially under mineralocorticoid control. Aldosterone and
other compounds with mineralocorti-coid activity bind to a specific
mineralocorticoid recep-tor in the cytoplasm of late distal tubule cells and of
principal cells of the collecting ducts. This hormone– receptor complex is
transported to the cell nucleus, where it induces synthesis of multiple
proteins that are collectively called aldosterone-induced proteins. The precise
mechanisms by which these proteins enhance NA+ transport are
incompletely understood. However, the net effect is to increase NA+ entry
across apical cell membranes and to increase basolateral membrane NA+
–K+ –ATPase activity and synthesis.
Mechanism of Action
Spironolactone (Aldactone) is structurally related to
aldosterone and acts as a competitive inhibitor to prevent the binding of
aldosterone to its specific cellular binding protein. Spironolactone thus
blocks the hormone-induced stimulation of protein synthesis necessary for NA+
reab-sorption and K+ secretion. Spironolactone,
in the presence of circulating
aldosterone, promotes a modest increase in NA+ excretion associated
with a decrease in K+ elimination. The observations that
spironolactone is ineffective in adrenalectomized patients and that the actions
of spironolactone can be reversed by raising circulating al- dosterone blood
levels (surmountable antagonism) sup-port the conclusion that spironolactone
acts by competi-tive inhibition of the binding of aldosterone with receptor
sites in the target tissue. Spironolactone
acts only when mineralocorticoids are
present.
Pharmacokinetic Properties
Spironolactone is poorly
absorbed after oral adminis-tration and has a delayed onset of action; it may
take sev-eral days until a peak effect is produced. It has a some-what slower
onset of action than triamterene and amiloride (discussed later), but its
natriuretic effect is modestly more pronounced, especially during long-term
therapy. Spironolactone is rapidly and extensively metab-olized, largely to the
active metabolite canrenone.
Canrenone and potassium canrenoate, its K+ salt, are available for
clinical use in some countries outside the United States. Canrenone has a
half-life of approximately 10 to 35 hours. The metabolites of spironolactone
are ex-creted in both the urine and feces. New selective aldos-terone receptor
antagonists (SARA), such as eplerenone, have been developed but have not yet
been introduced into clinical practice. Eplerenone and canrenone exhibit fewer
steroidlike side effects (gynecomastia, hirsutism).
Clinical Uses
Spironolactone has been used
clinically in the fol-lowing conditions:
·
Primary hyperaldosteronism. Used as an aid in preparing patients with adrenal
cortical tumors for surgery.
·
Hypokalemia. Used in patients with low
serum K+ resulting from diuretic therapy with other agents. Its use
should be restricted to patients who are un-able to supplement their dietary K+
intake or ad-equately restrict their salt intake or who cannot tolerate
orally available KCl preparations.
·
Hypertension and congestive
heart failure. Although spironolactone may be useful in com-bination with
thiazides, the latter remain the drugs of first choice. Fixed-dose combinations
of spironolactone and a particular thiazide (e.g., Aldactazide) generally offer no therapeutic ad-vantage over either
component given separately and tend to restrict the ability of the clinician to
determine the optimal dosage of each drug for a particular patient.
·
Cirrhosis and nephrotic
syndrome. Spironolactone is a mild
diuretic and may be useful in treating the edema that occurs in these two
clinical conditions, that is, when excessive K+ loss is to be
avoided.
Adverse Effects
Serum electrolyte balance
should be monitored peri-odically, since potentially fatal hyperkalemia may occur, especially in patients with impaired renal
function or ex-cessive K+ intake (including the K+ salts
of coadminis-tered drugs, e.g., potassium penicillin). Spironolactone can
induce hyponatremia and in cirrhotic patients, meta-bolic acidosis. A variety
of gastrointestinal disturbances may accompany spironolactone administration.
These include diarrhea, gastritis, gastric bleeding, and peptic ul-cers.
Spironolactone is contraindicated in patients with peptic ulcers.
Spironolactone may also cause elevated blood urea nitrogen, drowsiness,
lethargy, ataxia, confu-sion, and headache. Gynecomastia and menstrual
irreg-ularity in males and females, respectively, can occur. Painful gynecomastia (directly related to dosage
level and duration of therapy), which is generally reversible, may necessitate
termination of therapy. Animal studies demonstrating tumorigenic potential
support the clinical judgment that spironolactone alone or in combination
should not be used for most patients who require di-uretic therapy and its
unnecessary use should be avoided.
Nonsteroidal Potassium-sparing Drugs: Triamterene and Amiloride
Triamterene (Dyrenium) or amiloride (Midamor) ad-ministration results in
changes in urinary electrolyte patterns that are qualitatively similar to those
produced by spironolactone. The mechanism by which these agents bring about the
alterations in electrolyte loss, however, is quite different. Triamterene and amiloride produce their effects whether or not
aldosterone or any other mineralocorticoid is present. The action of these two drugs is clearly unrelated to
endogenous mineralo-corticoid activity, and these
drugs are effective in adrena-lectomized patients.
Mechanism of Action
Both agents appear to affect NA+
reabsorption in the cortical collecting duct. A site in the connecting tubule
also may be involved. Although amiloride has been more extensively studied than
triamterene, both diuretics specifically block the apical
membrane epithe-lial NA+ channel (ENaC) (Fig. 21-5). The reduced rate of NA+ reabsorption diminishes the gradient that
facili-tates K+ secretion. K+ secretion by the collecting
duct principal cells is a passive phenomenon that depends on and is secondary
to the active reabsorption of NA+ .
In addition to their effects
on distal NA+ and K+ transport, all of the K+ -sparing diuretics inhibit urinary H+ secretion by the late distal
tubule and cortical collect-ing duct. The mechanism of this inhibitory
action is not totally clear.
Pharmacokinetic Properties
Both triamterene and
amiloride are effective after oral administration. Diuresis ensues within 2 to
4 hours after administration, although a maximum therapeutic effect may not be
seen for several days. Both drugs cause a modest (2–3%) increase in NA+
and HCO3 -ex-cretion, a reduction in K+ and H+
loss, and a variable ef-fect on Cl- elimination. Approximately 80%
of an ad-ministered dose of triamterene is excreted in the urine as
metabolites; amiloride is excreted unchanged.
Clinical Uses
Triamterene can be used in
the treatment of conges-tive heart failure, cirrhosis, and the edema caused by
secondary hyperaldosteronism. It is frequently used in combination with other
diuretics except spironolactone. Amiloride, but not triamterene, possesses
antihyperten-sive effects that can add to those of the thiazides.
These K+ -sparing
diuretics have low efficacy when used alone, since only a small amount of total
NA+ re-absorption occurs at more distal sites of the nephron. These
compounds are used primarily in
combination with other diuretics, such as the thiazides and loop
di-uretics, to prevent or correct hypokalemia. The avail-ability of fixed-dose
mixtures of thiazides with nons-teroidal K+ -sparing compounds has
proved a rational form of drug therapy. Both triamterene and amiloride are
available alone or in combination with hy-drochlorothiazide.
Adverse Effects
Because the actions of
triamterene and amiloride are independent of plasma aldosterone levels, their
pro-longed administration is likely to result in hyper-kalemia. Both amiloride
and triamterene are con-traindicated in patients with hyperkalemia. Triamterene
should not be given to patients with impaired renal function. Potassium intake
must be reduced, especially in outpatients. A folic acid deficiency has been
reported to occur occasionally following the use of triamterene.
Related Topics