Chapter: Obstetrics and Gynecology: Contraception
Hormonal Contraceptives: Oral Contraceptives
HORMONAL CONTRACEPTIVES
For many women, “birth control”
is synonymous with oral contraceptives (OCs) or hormonal contraception. Hormone-dependent
choices now also include injectable hormonal preparations, an implantable
hormonal rod, con-traceptive patches, hormone-containing intrauterine sys-tems,
and contraceptive rings.
Oral Contraceptives
About one-third of all sexually active women in the United States use oral contraceptives, with over one half of young women 20 to 24 years old using these contraceptives.
Hormonal contraceptives have many
health benefits, including decreasing a woman’s risk of ovarian and uterine
cancer. Although hormonal contraceptive methods are associated with risks, for
most women the use of one of these agents is safer than pregnancy.
Hormone-based contraceptives
provide the most ef-fective reversible pregnancy prevention available. Method
(theoretical) failure rates for oral, transdermal, and trans-vaginal
contraceptives are in the range of ≤1%.
Longer-acting hormonal methods (injections, implants, and intrauterine
contraception) have effectiveness rates that equal or even surpass those of
sterilization. Because OC failures are usually related to missed pills,
injectable long-acting agents, patches, implants, intrauterine contracep-tion,
and rings share the additional advantage of lack of a need for daily
compliance.
Hormonal contraceptives do not
protect against STDs. Women who use these techniques should be counseled about
high-risk behaviors and the need to use condoms for additional protection.
MECHANISMS OF ACTION
Most of the oral contraceptives
are combinations of an estrogen and
a progestin, although there are also
progestin-only products. Combination oral contracep
The progestational component
pro-vides the major contraceptive effect, acting primarily by suppressing
secretion of luteinizing hormone, and, in turn, ovulation. The progestational
agent also provides the secondary effect of thickening the cervical mucus and
also alters fallopian tube peristalsis, inhibiting sperm move-ment and
fertilization, if ovulation were to occur. The estrogenic component acts by
suppressing secretion of follicle-stimulating hormone (FSH), preventing
matura-tion of a follicle, as well as by potentiation of the action of the
progestational agent. The estrogen provides an addi-tional modest contraceptive
effect, thus increasing the ef-ficacy of this method.
Common progestin compounds used
in hormonal contraceptives include, in descending order of biologic progestin
activity: norgestrel, ethynodiol diacetate, nor-ethindrone acetate,
norethynodrel, and norethindrone. Oral contraceptives using the less androgenic
agents, des-ogestrel, norgestimate, and drospirenone, are also available if
less androgenic activity is desired.
Many oral contraceptives contain
a fixed ratio of estro-gen and progestin, although “phasic” formulations have been introduced that vary this ratio
during the course of the month. This leads to a slight decrease in the total
dose of hormones used per month but is also associated with a slightly higher
rate of break-through bleeding
(bleeding not related to the menstrual period in a woman using oral
contraceptives) between periods.
The classic regimen for hormonal
contraception has been 21 days of active hormone (pill, patch, and ring) and 7
days of placebo or no hormones. Hormone regimens are also available that
produce shorter or less frequent menstrual periods. These new regimens shorten
the withdrawal bleed and decrease menstrual-related symptoms. Another
for-mulation is a monophasic ethinyl estradiol/levonorgestrel preparation that
extends the cycle to 3 months. Some women may prefer this usage pattern,
although they should be aware that there is a higher incidence of break-through
bleeding in the first 12-week cycle, compared with the 4-week cycle
preparations. New preparations continue to be developed with the ultimate goal
of maximizing benefits and minimizing side effects.
Progestin-only
contraceptives (progestin-only “mini-pill”) act primarily by
making the cervical mucus thick and relatively impermeable. Ovulation continues
normally in about 40% of patients using the progestin-only formu-lation. These
oral contraceptives are of special usefulness in two clinical situations:
lactating women and women over 40. In the former group, the progestin effect
co-incides with the prolactin-induced suppression of ovulation; in the latter
group, the inherent reduced fecundity adds to the progestin effect. There is no
effect on the quality or quantity of breast milk or any evidence of short- or
long-term adverse effects on infants, and the progestin-only pill may be
started immediately after delivery in the breast-feeding mother. The progestin-only pill is also a good
choicefor women in whom estrogen-containing formulations are con-traindicated. Because
of the low dosages of progestin, theminipill must be taken at the same time
each day, starting on the first day of menses. If a woman is more than 3 hours
late in taking the minipill, a back-up contraceptive method should be used for
48 hours.
EFFECTS OF HORMONAL CONTRACEPTIVES
Hormonal contraception affects
more than just the repro-ductive system. Estrogens
affect lipid metabolism, potentiatesodium and water retention, increase renin
substrate, stimulate the cytochrome P-450 system, increase sex hormone-binding
globulin, and can reduce antithrombin III. Progestins increasesebum,
stimulate the growth of facial and body hair, in-duce smooth muscle relaxation,
and increase the risk of cholestatic jaundice. The newer progestational agents—
desogestrel, norgestimate, and drospirenone—have less metabolic impact.
Oral contraceptives
have many beneficial effects. Menstrualperiods are predictable,
shorter, and less painful and, as a result, the risk of iron-deficiency anemia
is reduced. Oral contraceptive users have a lower incidence of endometrial and
ovarian cancers, benign breast and ovarian disease, and pelvic infection. By
decreasing conception, the risk of ec-topic pregnancy is reduced, along with
the complications of undesired intrauterine pregnancies.
Break-through bleeding occurs in
10% to 30% of women taking low-dose oral contraceptives during the first 3
months of use. Although it is an especially worri-some symptom, it is not
associated with decreased effi-cacy as long as the pill-taking regimen is
maintained. The abnormal bleeding pattern is the most common reason for
discontinuation of contraception and women should be counseled to expect
irregularities before hormones are initiated. If break-through bleeding does
occur, it is best managed by encouragement and reassurance, because it usually
resolves spontaneously. Break-through bleeding after approximately 3 months is
associated with progestin-induced decidualization, with the shallow and fragile
endometrium prone to asynchronous breakdown and bleeding. A short course of
exogenous estrogen (1.25 mg conjugated estrogen for 7 days), given while the
patient continues oral contraceptive use, usually stabilizes the endometrium
and stops the bleeding. Taking two or three of the pills each day is not an
effective therapy for break-through bleeding, because the progestin component
will predominate, often worsening the problem by causing further
decidualization of the endometrium.
Amenorrhea
occurs in approximately 1% of usersof low-dose oral
contraceptives in the first year of use, reaching perhaps 5% of users after several
years of use. Contraceptive efficacy is maintained if the pill regimen is
followed. Changing to a higher estrogen-containing pill or use of exogenous
estrogen may be employed to induce bleeding, if the patient wishes. A pregnancy
test should precede therapy.
Serious complications (such as
venous thrombosis, pulmonary embolism, cholestasis and gallbladder disease,
stroke, and myocardial infarction) are more likely for women using high-dose
formulations. However, these complications also can occur occasionally in
patients tak-ing low-dose formulations. Hepatic tumors have also been
associated with the use of high-dose oral contraceptives. Although all of these
complications are from 2 to 10 times more likely in pill-users, they are still
uncommon.
Less serious but more common side
effects also depend on the dosage and type of hormones used. Estrogens may
cause a feeling of bloating and weight gain, breast tenderness, nausea,
fatigue, or headache. Studies have demonstrated no overall weight gain in pill users
despite the perception of weight gain. Altering the dose or com-position of the
progestational agent used may relieve some of these minor side effects.
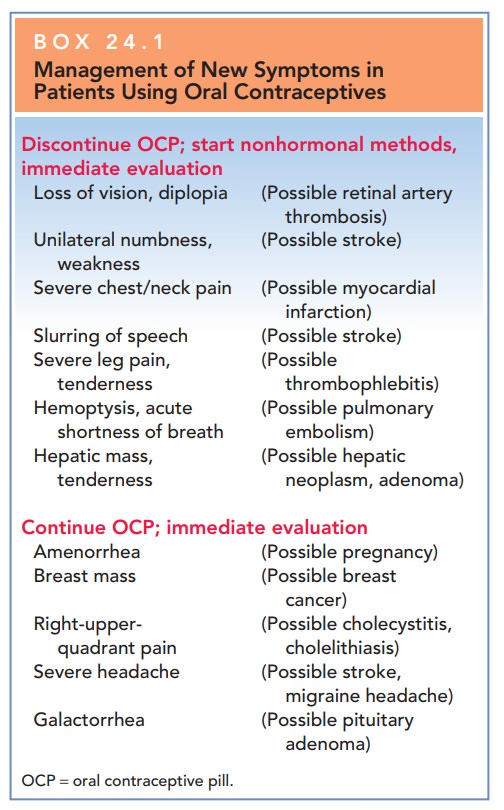
The therapeutic principle of contraception is to se-lect the method providing effective contraception with the greatest margin of safety, and then to use it as long as the patient wishes contraception or beneficial menstrual-related changes. If the patient experiences new signs or symptoms while using hormonal contraception, further evaluation or cessation of the chosen hormonal method may be required (Box 24.1).
PATIENT EVALUATION FOR COMBINED HORMONAL CONTRACEPTIVE USE
Before considering estrogen- and
progestin-containing contraceptives for a patient, a careful evaluation is
required. Not only are hormones relatively or absolutely contraindi-cated in
some patients, but also factors such as previous menstrual history may have an
impact on the choice of these agents. Combined oral conceptive use is
contraindi-cated in women over age 35 years who smoke or who have had a
thromboembolism, and in women with a history of coronary artery disease,
congestive heart failure, or cere-bral vascular disease (Box 24.2).
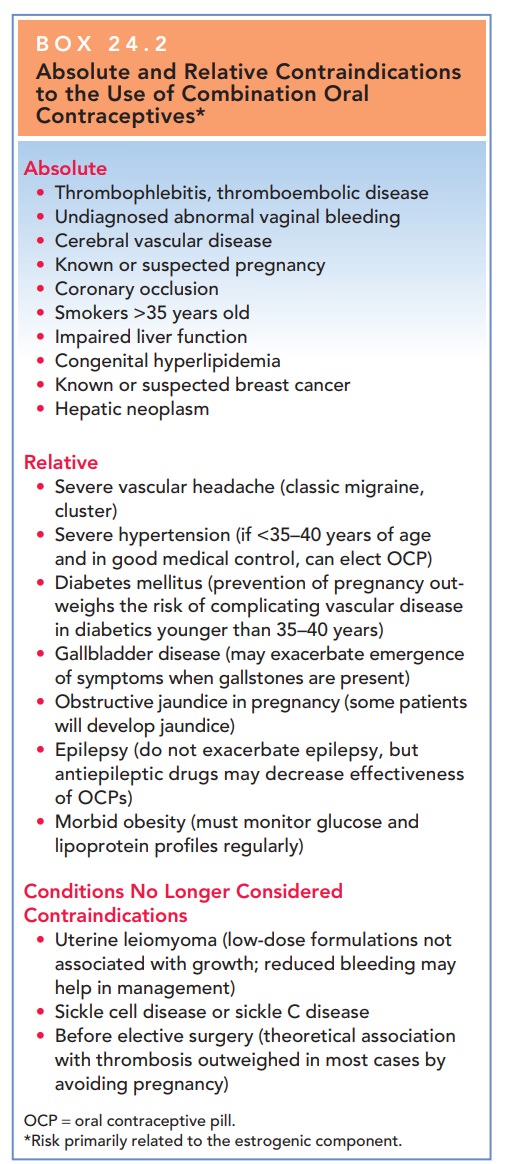
Box 24.2
Absolute and Relative Contraindications to the
Use of Combination Oral Contraceptives*
Absolute
Thrombophlebitis, thromboembolic disease
Undiagnosed abnormal vaginal bleeding
Cerebral vascular disease
Known or suspected pregnancy
Coronary occlusion
Smokers >35 years old
Impaired liver function
Congenital hyperlipidemia
Known or suspected breast cancer
Hepatic neoplasm
Relative
Severe vascular headache (classic migraine, cluster)
Severe hypertension (if <35–40 years of age and in good medical
control, can elect OCP)
Diabetes mellitus (prevention of pregnancy out-weighs the risk of
complicating vascular disease in diabetics younger than 35–40 years)
Gallbladder disease (may exacerbate emergence of symptoms when
gallstones are present)
Obstructive jaundice in pregnancy (some patients will develop jaundice)
Epilepsy (do not exacerbate epilepsy, but antiepileptic drugs may
decrease effectiveness of OCPs)
Morbid obesity (must monitor glucose and lipoprotein profiles regularly)
Conditions No Longer Considered Contraindications
Uterine leiomyoma (low-dose formulations not associated with growth; reduced
bleeding may help in management)
Sickle cell disease or sickle C disease
Before elective surgery (theoretical association with thrombosis outweighed in most cases by avoiding pregnancy)
OCP
= oral contraceptive pill.
*Risk
primarily related to the estrogenic component.
Approximately 3% of patients may
experience prob-lems with resumption of their periods after prolonged
contraceptive use (postpill amenorrhea).
Younger women and those who had irregular periods before the use of oral
contraceptives are more likely to experience this problem after discontinuing
their use. These patients should be counseled about this potential
complication.
Hormonal
contraceptives may interact with other medica-tions that the patient is taking.
This interaction may reducethe efficacy of either
the contraceptive or the other med-ications. Examples of drugs that decrease
the effectiveness of contraceptives include barbiturates, benzodiazepines,
phenytoin, carbamazepine, rifampin, and the sulfon-amides. Drugs that may show
retarded biotransformation when contraceptives are also used include
anticoagulants, methyldopa, phenothiazines, reserpine, and tricyclic
anti-depressants. Antibiotics may alter the intestinal flora and are thought to
interfere with hormone absorption, but ef-ficacy is not reduced. Before prescribing medications to womenusing
contraceptives, the clinician should consider possible drug interactions.
Related Topics