Chemical Kinetics - Half life period of a reaction | 12th Chemistry : UNIT 7 : Chemical Kinetics
Chapter: 12th Chemistry : UNIT 7 : Chemical Kinetics
Half life period of a reaction
Half
life period of a reaction:
The half life of a
reaction is defined as the time required for the reactant concentration to
reach one half its initial value. For a first order reaction, the half life is
a constant i.e., it does not depend on the initial concentration.
The rate constant for a
first order reaction is given by
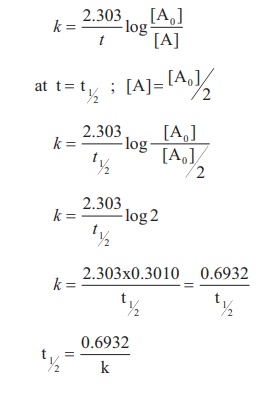
Let us calculate the half
life period for a zero order reaction.
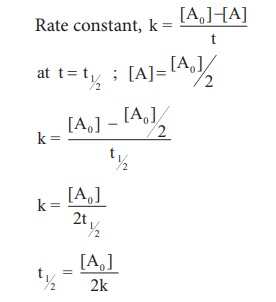
Hence, in contrast to the half life of a first order reaction, the half life of a zero order reaction is directly proportional to the initial concentration of the reactant.
Example
4
A first order reaction takes 8 hours
for 90% completion. Calculate the time required for 80% completion. (log 5 =
0.6989 ; log10 = 1)
Solution:
For a first order reaction,
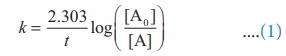
Let [A0 ] = 100M
When
t = t90% ; [A]=10M (given
that t90 % =8hours)
t = t80% ; [A]=20M
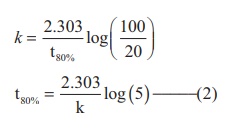
Find the value of k using the given
data
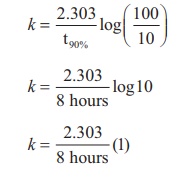
Substitute the value of k in
equation (2)
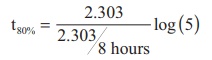
t80% = 8hours x 0.6989
t80% = 5.59hours
Example
5
(ii) The half life of a first order reaction
x → products is 6.932 x 104s at 500K . What percentage of x would be
decomposed on heating at 500K for 100 min. (e0.06 = 1.06)
Solution:
Given t1/2 = 0.6932 x 104
s
To solve :2 when t=100 min,
[ [A0 ] −[A] / [A0] ] x 100 = ?
We know that
For a first order reaction, t1/2
= 0.6932 / k
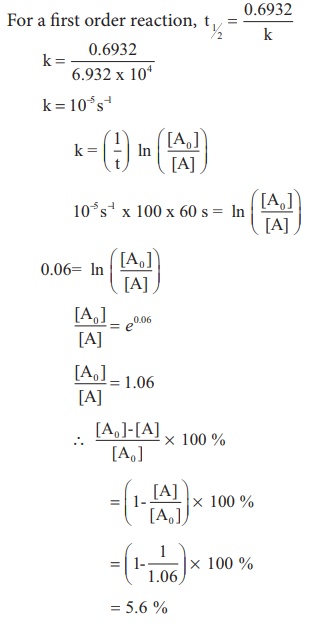
k = 10−5 s−1
Example
6
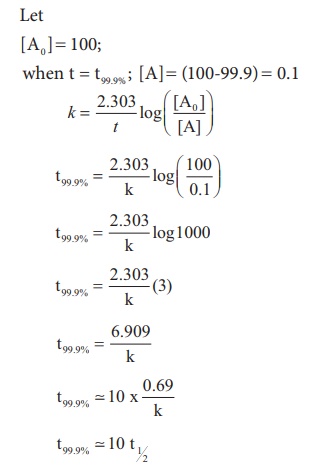
Show that in case of first order
reaction, the time required for 99.9% completion is nearly ten times the time
required for half completion of the reaction.
Evaluate
yourself:
1. In a first order reaction A → products 60% of the given sample of A
decomposes in 40 min. what is the half life of the reaction?
2. The rate constant for a first
order reaction is 2.3 X 10 −4 s−1 If the initial
concentration of the reactant is 0.01M . What concentration will remain after 1
hour?
3. Hydrolysis of an ester in an
aqueous solution was studied by titrating the liberated carboxylic acid against
sodium hydroxide solution. The concentrations of the ester at different time
intervals are given below.
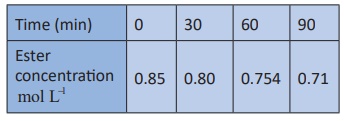
Show that, the reaction follows
first order kinetics.
Related Topics