Chapter: The Diversity of Fishes: Biology, Evolution, and Ecology: Conservation and the future of fishes
Habitat loss and modification - General causes of fish biodiversity decline
Habitat loss and modification
Human alteration of aquatic habitats is the most commonly cited cause of declines in fish populations. Habitats are altered via modification of bottom type and above-bottom structure – channelization, dam building, watershed perturbation, and competition for water.
Modification of bottom type
Many fish species are ecologically dependent on bottom topography and above-bottom structure for successful survival. In flowing water, rocks and logs provide shelter from the current and a site of attachment for eggs, algae, and associated fauna. Undersides of rocks are a major refuge for insect larvae and other invertebrates that fishes eat. Aquatic vegetation similarly provides shelter and food attachment sites for lacustrine fishes. In the ocean, rocks and biogenic habitat (corals, sponges, many other sessile invertebrates, kelp beds and other attached algae) are essential habitat for most benthic species. Human activities that disrupt, remove, or cover bottom structure will be detrimental to fishes. Such activities include dredging for navigation and to obtain construction materials, bottom trawling in the ocean, removal of logs and debris dams to aid navigation and as “habitat improvement”, and watershed disruption that leads to increased erosion and silt deposition (Fig. 26.2).
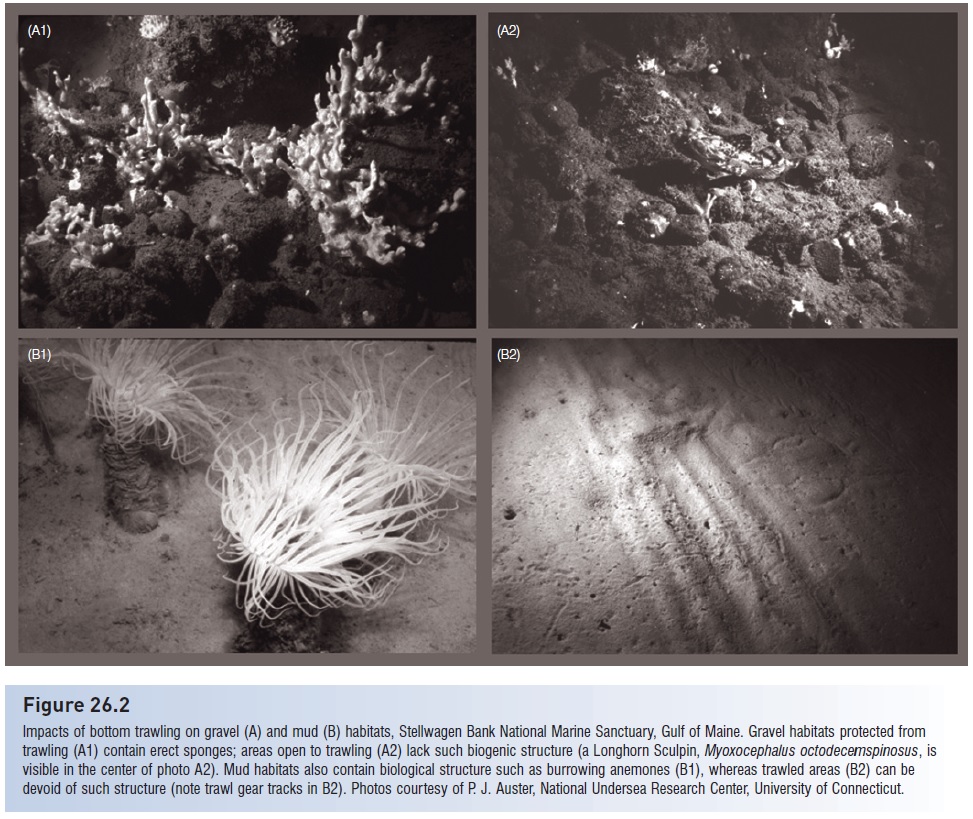
Figure 26.2
Impacts of bottom trawling on gravel (A) and mud (B) habitats, Stellwagen Bank National Marine Sanctuary, Gulf of Maine. Gravel habitats protected from trawling (A1) contain erect sponges; areas open to trawling (A2) lack such biogenic structure (a Longhorn Sculpin, Myoxocephalus octodecemspinosus, is visible in the center of photo A2). Mud habitats also contain biological structure such as burrowing anemones (B1), whereas trawled areas (B2) can be devoid of such structure (note trawl gear tracks in B2). Photos courtesy of P. J. Auster, National Undersea Research Center, University of Connecticut.
Woody debris in streams and rivers exemplifi es the effects of habitat disruption on fishes. Woody debris, in the form of debris dams in streams and of logs (=snags) in rivers, plays a critical role in ecosystem function (Wallace & Benke 1984; Harmon et al. 1986; Maser & Sedell 1994). Debris dams retain silt, organic matter, and nutrients, offer a solid substrate for invertebrate attachment, and are a site for transformation and processing of organic matter, thus making it available for invertebrate and fish use. Woody debris also slows the flow of the water, which decreases erosion and increases the time during which nutrients are available to the food web. In coastal, low-gradient (slow moving) rivers, many gamefishes obtain more than half of their food directly from snags. Snags are the most biologically rich habitat in such rivers: although making up only 4% of habitable surfaces, snags contain 60% of the total invertebrate biomass, provide 80% of the drifting invertebrate biomass, and produce four times more prey than mud or sand habitats (Benke et al. 1985). Government efforts at snag removal in navigable rivers of the southeastern USA began in the early 1800s. When rail transportation largely replaced river commerce in the 1850s, snag removal was
In the tropics, catastrophic deforestation along rivers and streams adversely modifi es both terrestrial and aquatic habitats (Chapman & Chapman 2003). In tropical marine environments, coral reef destruction occurs at an equally alarming rate. Coral reefs contain the most diverse fish assemblages on earth, but reefs suffer both directly and indirectly from human activities. Habitats are destroyed by the direct mining and collecting of coral, and inadvertently by harmful fishing techniques (poisons, explosives, bottom trawling), boat anchoring and diver activities, sedimentation and pollution, boat groundings, and changes in coral predator abundance as a result of fishing practices. All these phenomena lead to reductions in fish diversity and biomass because fishes and their prey rely directly on corals for food and shelter (Birkeland 1997).
Coral mining is a particularly deleterious activity. Limestone blocks are cut from the reef surface and then used in road and home building and as landfill. Massive, headforming corals in shallow (1–2 m depth) water are most frequently targeted. Where heavily practiced, coral cover can change from 50% to 5% both as a direct result of removal and as a byproduct of trampling and sediment production. Recovery is slow, taking more than a decade, if it occurs at all. Fish biomass, abundance, and diversity decline in mined areas through reduction in living coral and also through reduction in substrate rugosity (topographic complexity) (Bell & Galzin 1984; Shepherd et al. 1992).
Coral collecting for the aquarium trade has also taken a significant toll on reef habitat (Derr 1992). Both live corals
and algae-covered or invertebrate-encrusted dead corals are taken. Live coral and “live rock” were removed from the Florida Keys at a rate of 3 tons/day in 1989, with an annual retail value of around U$10 million. Live rock consists of substrate built over 4000–7000 years and does not represent a renewable resource on the reef. Mortality rates for live corals in aquaria exceed 98% within 18 months of collection. Because of the acknowledged difficulties of keeping live coral and reef-building invertebrates in captivity, most large, commercial “public” aquaria use artifi cial corals; home aquarists should do the same.
Channelization
Channelization, also referred to as “bank stabilization”, involves straightening a riverine system and smoothing its sides. Bends in a river are bulldozed into straight lines, levees are built, and banks are covered and heightened with stones and boulders (riprap) or concrete. Rivers and streams are channelized primarily to reduce seasonal inundation of the floodplain (so-called because the floodplain is the natural area that receives overflow during seasonal rains); channelization is basically the process by which a river or stream is converted into a ditch or pipe. Channelized stream segments have low habitat heterogeneity and higher velocities during higher flows. Shallow water and floodplain habitats are eliminated, both of which provide spawning and nursery areas for riverine fishes. Channelized rivers either lack fishes or are dominated by introduced species. Especially affected are big river species, species dependent on sandy areas, and fishes that use the floodplain in their life cycle, including sturgeons, Paddlefish, and darters of the genus Ammocrypta. Channelization-induced loss of the floodplain in parts of the Lower Mississippi River has led to a 10-fold reduction in standing biomass of fishes.
Because channelization is often accompanied by deforestation of the floodplain to allow for agriculture and housing development, the entire hydrological regime of a river is altered, with a result that flooding actually increases (Simpson et al. 1982; Moyle & Leidy 1992). The catastrophic flooding of the Mississippi River in 1993 was partly due to decades of channelization (Myers & White 1993); inundation of New Orleans by Hurricane Katrina in 2005 was a result of levee construction that delivered sediment too far downstream thus preventing the development of wave-buffering, nearshore wetlands.
The adverse effects of channelization have been so great in some areas that expensive “dechannelization” programs have been initiated. In southern Florida, the US Army Corps of Engineers channelized the meandering, shaded, productive 165 km long Kissimmee River, turning it into a 90 km long, straight, concrete canal. Channelization resulted in drained wetlands (including desiccation of substantial portions of the Everglades National Park), water pollution and eutrophication, periodic flooding, salt contamination of streams and aquifers, water table lowering and land subsidence, oxidation of peat soils, wind erosion, and marsh fi res. Biological effects included a 90% decrease in wading bird populations, the deaths of 5 billion fishes and 6 billion shrimp, and extirpation of six native fishes from the Kissimmee River. In 1976, Florida reconsidered the project. The Corps proposed dechannelization, which began with feasibility studies in 1978–85 and then again in 1990–98. Actual dechannelization is ongoing and will constitute the largest river restoration project ever undertaken in the USA, requiring more than 13 years and costing over $500,000,000 (this number increases regularly). Restoring the river, which has begun, will take several years more and cost perhaps 10 times more than channelization (Koebel 1995; Whalen et al. 2002).
Dam building
Dams provide hydroelectric power, water storage capacity (although evaporation often minimizes water storage benefits in arid regions), agricultural water, recreational opportunities, and lakefront development potential. Drawbacks of dam building include flooding of agriculturally and historically valuable land. Poor watershed management, often brought on by deforestation of the land surrounding newly created reservoirs, leads to rapid silting-in of the lake, transforming it into a much less desirable (from a development standpoint) marsh or swamp. In tropical countries, regions around dams become uninhabitable for humans because the altered habitats favor organisms that cause such debilitating parasitic diseases as schistosomiasis and onchocerciasis (river blindness). Increases in these and other diseases are well documented in human populations residing near newly created dams (e.g., Steinmann et al. 2006).
The altered hydrological conditions behind dams can cause other, unforeseen problems. Piranha attacks on healthy humans in the Amazon Basin are exceedingly rare (Sazima & Guimaraes 1987). Dam construction created year-round, favorable, still-water conditions for piranha spawning, whereas spawning habitat was previously limited to flooded forest lands during the wet season. Piranhas defend their nest sites against intruders, including waders in the shallow waters of reservoirs. Single bite attacks characteristic of nest defense rose dramatically after dam construction. Bathers in reservoirs in the Parana–Paraguay river systems in southeast Brazil reported more than 85 piranha attacks on humans in 2002; 90% of bites were on the legs and feet, suggestive of defensive attacks on wading bathers by nest-guarding adults (Haddad & Sazima 2003). Wounds were crater-like, 1–2.5 cm in diameter, and bled severely. Several bites required hospitalization and one resulted in amputation of a toe.
Not too surprisingly, fishes adapted to flowing water do not fare well in the impounded regions behind dams. Many productive cold water trout fisheries have been lost behind dam walls. Stream assemblages, usually rich in native darters, minnows, suckers, and trouts, are usually replaced by sunfishes and catfishes. As is the case in most disturbed habitats, introduced species come to dominate, including Carp, Yellow Perch, Mosquitofish, and lacustrine minnows. The history of the Snail Darter serves as a good example of the biological and political complexities of dam building.
Two North American examples typify the effects of dams on aquatic faunas. The Colorado River was an ancient, warm, fast-flowing, turbid river that developed a unique fauna of streamlined fishes adapted to high flows and high temperatures. These fishes spawned in response to seasonal changes in water level and temperature. Of 32 fishes native to the Colorado River about 75% are endemic. More than 100 dams were built along this huge desert river for water retention, flood control, and agriculture; less than 1% of the virgin flow now reaches the river’s mouth. The deep reservoirs formed by many dams became thermally stratified, and water released periodically from the cold, lower portions of the reservoirs chilled downstream habitats, disrupting natural spawning cycles and killing native fishes while promoting the survival of introduced cold water predators such as Rainbow Trout. Of the 80 fish species that now occur in the Colorado River, only about one-third are native. Of the remaining native fishes, most are Threatened or Endangered, including the Humpback Chub (Gila cypha), the Bonytail Chub (G. elegans), the Razorback Sucker (Xyrauchen texanus), and the Colorado Pikeminnow (Ptychocheilus lucius), the largest minnow native to North America (Fig 26.3). The modified environment created by the dams and the success of introduced fishes are chief contributors to the decline of native fishes (Ono et al. 1983; Minckley 1991; Wydoski & Hamill 1991).
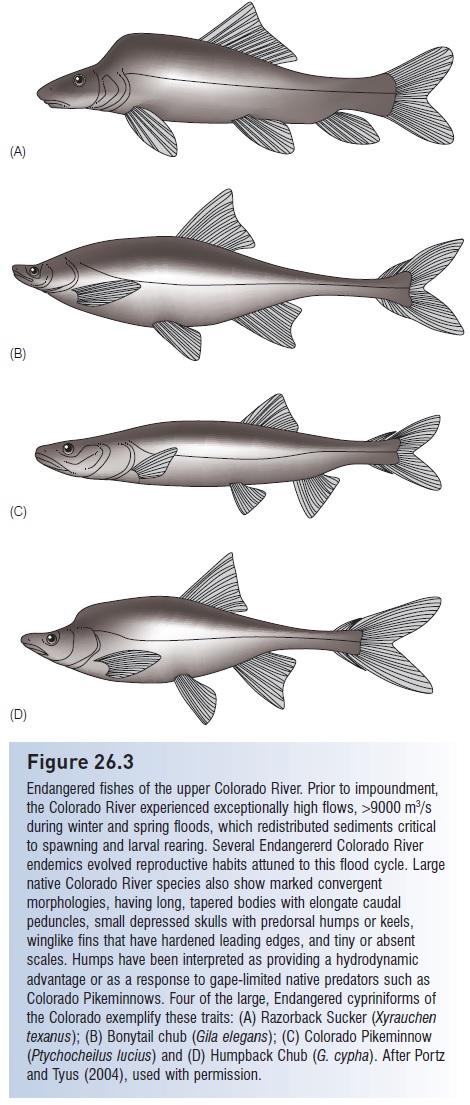
Figure 26.3
Endangered fishes of the upper Colorado River. Prior to impoundment, the Colorado River experienced exceptionally high flows, >9000 m3/s during winter and spring floods, which redistributed sediments critical to spawning and larval rearing. Several Endangererd Colorado River endemics evolved reproductive habits attuned to this flood cycle. Large native Colorado River species also show marked convergent morphologies, having long, tapered bodies with elongate caudal peduncles, small depressed skulls with predorsal humps or keels, winglike fins that have hardened leading edges, and tiny or absent scales. Humps have been interpreted as providing a hydrodynamic advantage or as a response to gape-limited native predators such as Colorado Pikeminnows. Four of the large, Endangered cypriniforms of the Colorado exemplify these traits: (A) Razorback Sucker (Xyrauchen texanus); (B) Bonytail chub (Gila elegans); (C) Colorado Pikeminnow (Ptychocheilus lucius) and (D) Humpback Chub (G. cypha). After Portz and Tyus (2004), used with permission.
Hydroelectric dams also block movements of fishes that migrate upriver to spawn, and pulverize juveniles during their downstream movements (Lucas & Baras 2001). Fishes that make it past tailwaters or through turbines often suffer from gas-bubble disease, brought on because the agitated waters below a dam are often supersaturated with gas (e.g., Raymond 1988). Habitat destruction, water flow reduction, and other dam effects are considered major factors causing the decline of salmonid stocks in western North America. The Columbia River system, including its large tributary Snake River, has a gauntlet of 28 dams that must be run by spawning adult salmonids and oceanbound juveniles. Upstream mortality is estimated at 5% and downstream mortality at 20% per dam (Booth 1989); four of the Columbia and Snake river dams lack any fish bypass structures such as fish ladders.
Commercial catches of salmon in the Columbia have declined dramatically (Fig. 26.4). For related reasons, approximately 106 major West coast salmon and steelhead stocks (Oncorhynchus spp.) have already been extinguished and an additional 214 native, naturally spawning stocks of Pacific salmons, Steelhead, and sea-run Cutthroat Trout are at risk in Oregon, California, Washington, and Idaho. Some analyses put the numbers as high as 280 stocks extinguished and another 880 stocks at high risk of extinction (Nehlsen et al. 1991; Huntington et al. 1996; Slaney et al. 1996).Overfishing, deforestation, hatchery introductions, aquaculture escapes, introduced pathogens, and agricultural and industrial pollution also contribute to the problem (NRC 1996b; Lichatowich 1999; Williams 2006; among many others).
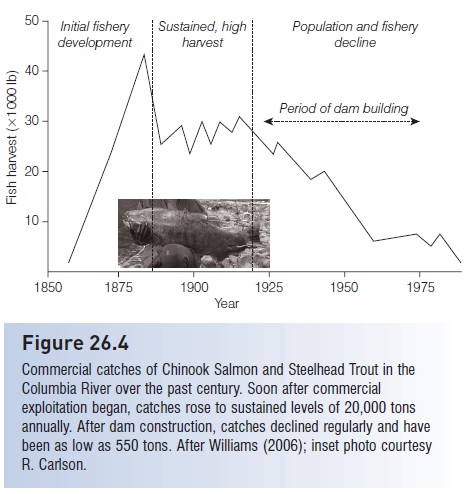
Figure 26.4
Commercial catches of Chinook Salmon and Steelhead Trout in the Columbia River over the past century. Soon after commercial exploitation began, catches rose to sustained levels of 20,000 tons annually. After dam construction, catches declined regularly and have been as low as 550 tons. After Williams (2006); inset photo courtesy R. Carlson.
Retention of sediments in reservoirs, combined with elimination of flood cycles, can have far-reaching consequences for fish production. Nutrients that would have been delivered to estuaries or dispersed over many kilometers of downstream floodplain during seasonal inundation remain trapped behind a dam. Construction of three dams in northern Nigeria led to a 50% reduction in downstream fish landings. Similar effects of dams have been reported in Zambia, South Africa, Ghana, and Egypt. In Egypt, con struction of the Aswan High Dam, which impounds 50– 80% of the Nile River’s flow, caused a 77% reduction in annual landings of sardines, Sardinella aurita, in the southeastern Mediterranean (Smith 2003). In eastern Europe, dams along the Volga River contributed to a 90% reduction in fish catches in the Caspian Sea. Similar, or worse, scenarios have been created in the Azov, Black, and Aral seas (Welcomme 1985; Moyle & Leidy 1992; Pringle et al.2000; see below).
Many options exist that will prevent, minimize, and reverse the negative impacts of dams. Dam construction, which is costly in addition to being environmentally destructive, can be avoided via conservation measures such as improving irrigation methods and other practices that reduce water loss, and conserving energy and developing alternative energy sources. Activities that reduce the impacts of existing dams include dam operation schedules that restore natural flows in river ecosystems, correcting sediment transport and deposition problems, correcting fish passage and entrainment problems, and, ultimately, removing dams that have outlived their usefulness (Heinz Center 2002). Dams modify entire ecosystems, more so than many of the other insults that humans visit on aquatic habitats and fishes (Dudgeon 2000). Correcting the damage requires an ecosystem perspective and ecosystem-level management actions.
Watershed perturbation
Aquatic systems include not only the water in which fishes live but also the groundwater and the surrounding landscape or terrestrial area through which water must flow. Many activities have an adverse effect on a river’s watershed (the land from which water drains into a river), including logging or burning of vegetation, bulldozing for construction and development, groundwater and surface water withdrawal and contamination, overgrazing and trampling of streamside vegetation, and erosion caused by wind, water, or the movements of livestock.
Much has been written about deforestation in tropical and temperate regions. Riparian trees, those that grow along stream and river banks, interact intimately with nearby water courses. Obvious consequences of tree removal include a rise in water temperature from loss of shade (from direct heating of a stream and transfer of heat to groundwater by irradiated soil), increased variation in flow rates because water uptake by plants is lost, intensified erosion leading to turbidity, siltation and stream bank collapse (particularly where logging operations occur on steep slopes), and loss of nutrient inputs from falling leaves and fruit.
Shade also reduces ultraviolet (UV) radiation. Fishes can suffer directly from UV exposure, including being sunburned (see Blazer et al. 1997), and some sun-dwelling fishes are even protected by mucus that has a sunscreen function (Zamzow & Losey 2002). Eggs, embryos, and larvae of marine and freshwater species suffer higher mortality when exposed to high but natural levels of solar UV-B (reviewed in Häkkinen et al. 2002). Excessive exposure to solar radiation induces cataracts in Rainbow Trout lenses (Cullen & Monteith-McMaster 1993), which diminishes a trout’s ability to focus images on the retina. Young of several fish species avoid UV light if refuges are available (Kelly & Bothwell 2002; Ylonen et al. 2004).
Siltation of streams is a major problem – it hinders productivity because of light reduction; eliminates refuge sites; decreases water clarity which makes sight feeding more difficult; depresses spawning activity; and smothers eggs, sessile invertebrates, and plants (Sutherland 2007; Sutherland & Meyer 2007). Silt and sediment are highly abrasive and cause loss of gill function, especially in juvenile fishes (Fig. 26.5). Siltation has been directly linked to native fish declines in many habitats, including Sri Lankan streams and South African estuaries (Moyle & Leidy 1992).
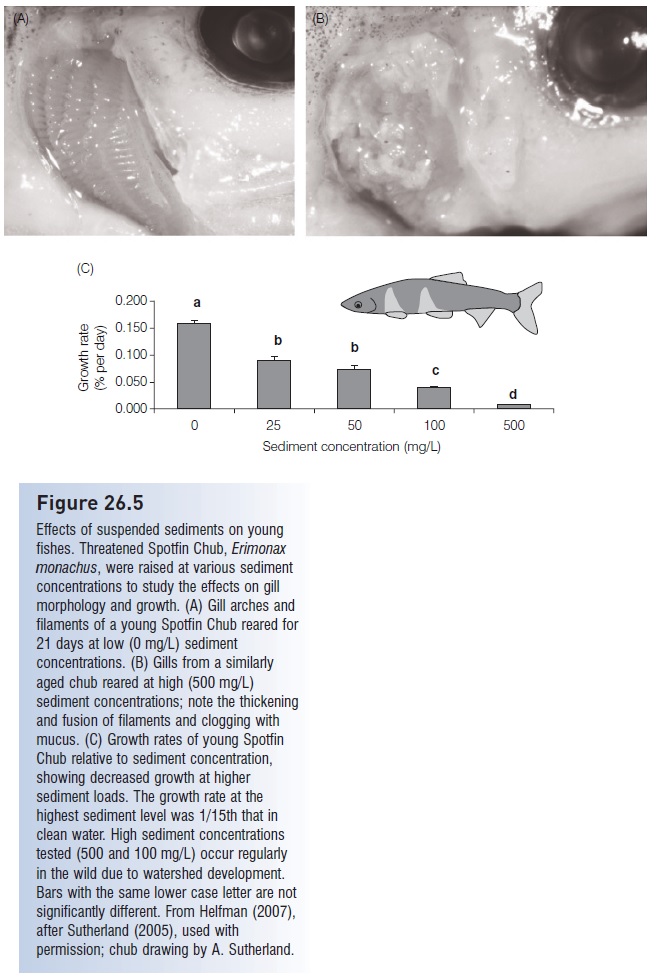
Figure 26.5
Effects of suspended sediments on young fishes. Threatened Spotfin Chub, Erimonax monachus, were raised at various sediment concentrations to study the effects on gill morphology and growth. (A) Gill arches and filaments of a young Spotfin Chub reared for 21 days at low (0 mg/L) sediment concentrations. (B) Gills from a similarly aged chub reared at high (500 mg/L) sediment concentrations; note the thickening and fusion of filaments and clogging with mucus. (C) Growth rates of young Spotfin Chub relative to sediment concentration, showing decreased growth at higher sediment loads. The growth rate at the highest sediment level was 1/15th that in clean water. High sediment concentrations tested (500 and 100 mg/L) occur regularly in the wild due to watershed development.
Bars with the same lower case letter are not significantly different. From Helfman (2007), after Sutherland (2005), used with permission; chub drawing by A. Sutherland.
Sedimentation is the largest source of contamination in North American streams and rivers (Waters 1995; USEPA 2000) and is the most important factor limiting the availability of fish habitat. Waters (1995, p. 79) stated that fine sediments constituted “perhaps the principal factor . . . in the degradation of stream fisheries”.
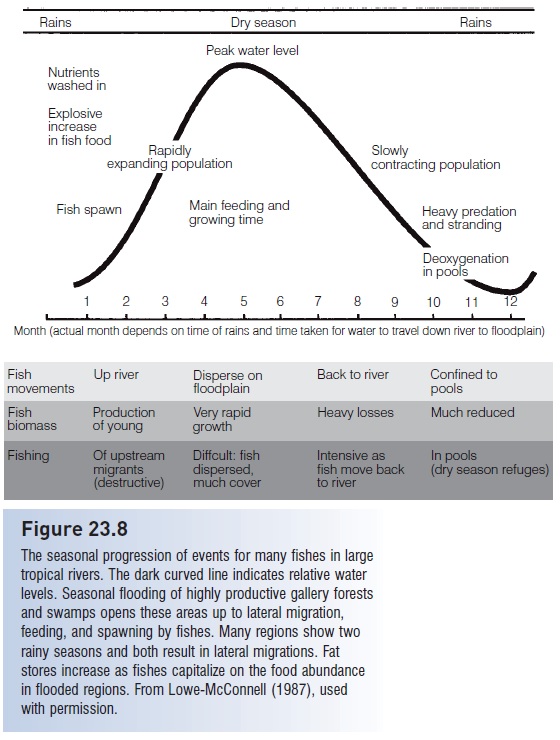
Another adverse effect of deforestation on aquatic systems involves the cessation of inputs of woody debris, in the form of branches and trunks that normally fall into a water course. Such structure is crucial to the productivity of many low-gradient rivers along coastal plains (see above). Many species use the exteriors and hollowed interiors of logs as spawning sites (e.g., catfishes, Ictaluridae) or as resting sites (Lowe-McConnell 1987). The gallery forests that line lowland rivers are also major spawning sites for fishes that migrate into their flooded zones during winter or spring floods at high latitudes and during rainy seasons at low latitudes (see Reproductive seasonality). The strong dependence of Amazonian fishes on seasonally inundated floodplains underscores the more general problem of wetland loss through logging and filling (Goulding 1980; see Fig. 23.8).
Logging along stream courses can have quite unexpected, complicated impacts on fish populations. Clearcutting in the Carnation Creek watershed of British Columbia raised stream temperatures 1–3°C. Elevated temperatures caused early emergence and accelerated growth of young Coho Salmon, Oncorhynchus kisutch. Smolts migrated earlier than normal and then experienced poor ocean survival, probably because their early arrival in the ocean placed them out of synchrony with prey cycles (Holtby 1988). Logging operations high in a watershed can affect ecosystem processes at distances far removed from the actual site of disturbance, such as when increased erosion causes unnaturally high levels of sediment deposition in coastal lagoons and estuaries (Moyle & Leidy 1992).
Competition for water
Humans use water for drinking, agriculture, recreation, fishing, and waste disposal. All these activities have adverse effects on aquatic organisms. Consumption and irrigation necessitate water withdrawals, leading to flow reductions in aquatic systems. Pumping of groundwater lowers water tables, which reduces the output of springs and seeps that are often necessary for maintaining year-round flow in many systems. Habitats subjected to withdrawals shrink, progressively losing heterogeneity and species. Downstream systems from which water is diverted evaporate, concentrating salts and pollutants. The universal use of waterways and waterbodies as dumping grounds for human waste creates environments toxic to fishes and humans.
Water withdrawal for irrigation of arid regions has created numerous ecological disasters, leading to species extinctions among fishes and other biota, and eventually producing salinated croplands and contaminated water supplies for humans. The history of species extinctions in the desert southwest of North America, summarized briefly above, serves as one example. At a larger scale are the events surrounding desiccation of the Aral Sea in the Uzbekistan/Kazakhstan region of the former Soviet Union. In 1960, the Aral Sea was the fourth largest lake in the world, covering 68,000 km2; it supported large commercial fisheries as well as extensive hunting in its wetlands. Inputs are primarily from river flow, losses are due to evaporation. Construction of diversion canals and withdrawal of water from its two major input rivers for irrigation purposes shrank the lake to only 41,000 km2 in 1987. By 1998, lake volume was reduced 80% from its original size. Lake salinity rose to 50 ppt in the 1990s, well above that of sea water, which is only 37 ppt. An original native fish fauna of 24 species has been reduced to four introduced species (Zholdasova 1997); commercial fisheries fell from 48,000 metric tons in 1957 to zero by the early 1980s.
Impacts have extended far beyond the ichthyofauna. Dust and salt storms, detectable on satellite imagery, originate on the dry lake bed and distribute 43 million metric tons (mmt) of crop-destroying salt annually over a 200,000 km2 area. Reduced river flow, salinization, pollution of remaining water, and lowering of the water table have led to a high incidence of intestinal illnesses, throat cancer, tuberculosis, and anemia, high infant mortality, and a death rate from respiratory ailments that ranks among the highest in the world. Economic losses of approximately 2 billion rubles (=U$3.2 billion) annually have been estimated for the Aral Sea region as a result of its desiccation (Micklin 1988). The Aral Sea disaster has been called “perhaps the most notorious ecological catastrophe of human making” (Stone 1999, p. 30).
Related Topics