Chapter: Medical Microbiology: An Introduction to Infectious Diseases: Streptococci and Enterococci
Group A Strepto Coccal Disease
GROUP A STREPTO COCCAL DISEASE
Group A streptococci are the cause of “strep throat,” an acute inflammation of the pharynx and tonsils that includes fever and painful swallowing. Skin and soft tis-sue infections range from the tiny skin pustules called impetigo to a severe toxic and invasive disease that can be fatal in a matter of days. In addition to acute infections, group A streptococci are responsible for inflammatory diseases that are not direct infections but represent states in which the immune response to streptococcal antigens causes injury to host tissues. Acute rheumatic fever (ARF) is a prolonged febrile inflammation of connective tissues, which recurs following each subsequent streptococcal pharyngitis. Repeated episodes cause permanent scarring of the heart valves. Acute glomerulonephritis is an insidious disease with hypertension, hematuria, proteinuria, and edema due to inflammation of the renal glomerulus.
EPIDEMIOLOGY
Pharyngitis
Group A streptococci are the most common bacterial cause of pharyngitis in school-age children 5 to 15 years of age. Transmission is person to person from the large droplets produced by infected persons during coughing, sneezing, or even conversation. This droplet transmission is most efficient at the short distances (2 to 5 feet) at which social in-teractions commonly take place in families and schools, particularly in fall and winter months. Asymptomatic carriers ( <1%) may also be the source particularly if colonized in the nose as well as the throat. Although group A streptococci survive for some time in dried secretions, environmental sources and fomites are not important means of spread.Unless the condition is treated, the organisms persist for 1 to 4 weeks after symptoms have disappeared.
Impetigo
Impetigo occurs when transient skin colonization with group A streptococci is combined with minor trauma such as insect bites. The tiny skin pustules are spread locally by scratching and to others by direct contact or shared fomites such as towels. Impetigo is most common in summer months when insects are biting and when the general level of hygiene is low. The M protein types of S. pyogenes most commonly associated with im-petigo are different from those causing respiratory infection.
Wound and Puerperal Infections
Group A streptococci, once a leading cause of postoperative wound and puerperal in-fections, retain this potential, but these conditions are now less common. As with staphylococci, transmission from patient to patient is by the hands of physicians or other medical attendants who fail to follow recommended handwashing practices. Organisms may be transferred from another patient or come from the health care work-ers themselves.
Streptococcal Toxic Shock Syndrome
Since the late 1980s, a severe invasive form of group A streptococcal soft tissue infection appeared with increased frequency (5 to 10 cases/100,000) in the United States and other countries. Rapid progression to death in only a few days occurred in previously healthy persons, including Muppet creator Jim Henson (of Sesame Street fame). The outstanding features of these infections are their multiorgan involvement suggesting a toxin and rapid invasiveness with spread to the bloodstream and distant organs. The toxic features to-gether with the discovery that almost all the isolates produce one of the SPEs have caused this syndrome to be labeled streptococcal toxic shock syndrome (STSS)
Poststreptococcal Sequelae
The association between group A streptococci and the inflammatory disease acute rheumatic fever (ARF; see the text) is based on epidemiologic studies linking group A streptococcal pharyngitis, the clinical features of rheumatic fever, and heightened im-mune responses to streptococcal products. ARF does not follow skin or other nonrespira-tory infection with group A streptococci. Although some M types may be more “rheumatogenic,” it is generally believed that recurrences of ARF can be triggered by in-fection with any group A streptococcus. Injury to the heart caused by recurrences of ARF leads to rheumatic heart disease, a major cause of heart disease worldwide. Although ARF has declined in developed countries ( <0.5 cases/1000), a resurgence in the form of small regional outbreaks in the United States began in the late 1980s. These outbreaks in-volved children of a higher socioeconomic status than previously associated with ARF and a shift in prevalent M types. The underlying basis of the resurgence is unknown.
Poststreptococcal glomerulonephritis may follow either respiratory or cutaneous group A streptococcal infection and involves only certain “nephritogenic” strains. It is more common in temperate climates where insect bites lead to impetigo. The average la-tent period between infection and glomerulonephritis is 10 days from a respiratory infec-tion, but generally about 3 weeks from a skin infection. Nephritogenic strains are limited to a few M types and seem to have declined in recent years.
PATHOGENESIS
Acute Infections
As with other pathogens, adherence to mucosal surfaces is a crucial step in initiating disease. A dozen adhesins have been described that facilitate the ability of the group A streptococcus to adhere to epithelial cells of the nasopharynx and/or skin. Of these, the most important are M protein, LTA, and protein F. In the nasopharynx, all three appear to be involved in mediating attachment to the fatty acid – binding sites in the glycoprotein fibronectin covering the epithelial cell surface. The role of M protein is not direct but it appears to provide a scaffold for LTA, which is essential for it to reach its binding site (Fig 17 – 3).
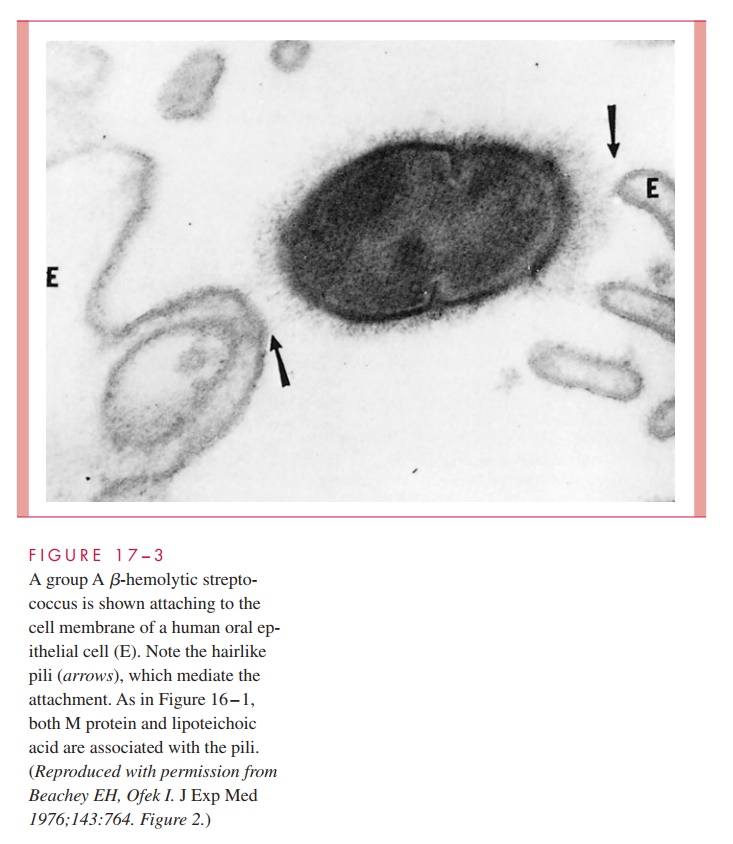
On the other hand, M protein appears to be direct and dominant in binding to the skin through its ability to interact with subcorneal keratinocytes, the most numerous cell type in cutaneous tissue. This adherence takes place at domains of the M protein that bind to CD46 and possibly other receptors on the keratinocyte surface. Protein F is also involved primarily in adherence to antigen-presenting Langerhans cells. Expression of M protein and protein F is environmentally regulated in response to changing concentrations of O2 and CO2. Experimental evidence suggests that a high O2 environment favors protein F and adherence to Langerhans cells, while an environment richer in CO2 favors M protein synthesis and interaction with keratinocytes. This environmentally controlled sequential interaction of S. pyogenes with different types of host cells should play some mitigating role either in establishing the microbe or in altering the development of a normally pro-tective host response.
Clinical evidence makes it clear that group A streptococci have the capacity to be highly invasive. The events following attachment that trigger invasion are only starting to be understood. It appears that M protein, protein F, and other fibronectin-binding proteins are required for invasion of nonprofessional phagocytes. This invasion involves integrin receptors and is accompanied by cytoskeleton rearrangements but the molecular events do not yet make a coherent story.
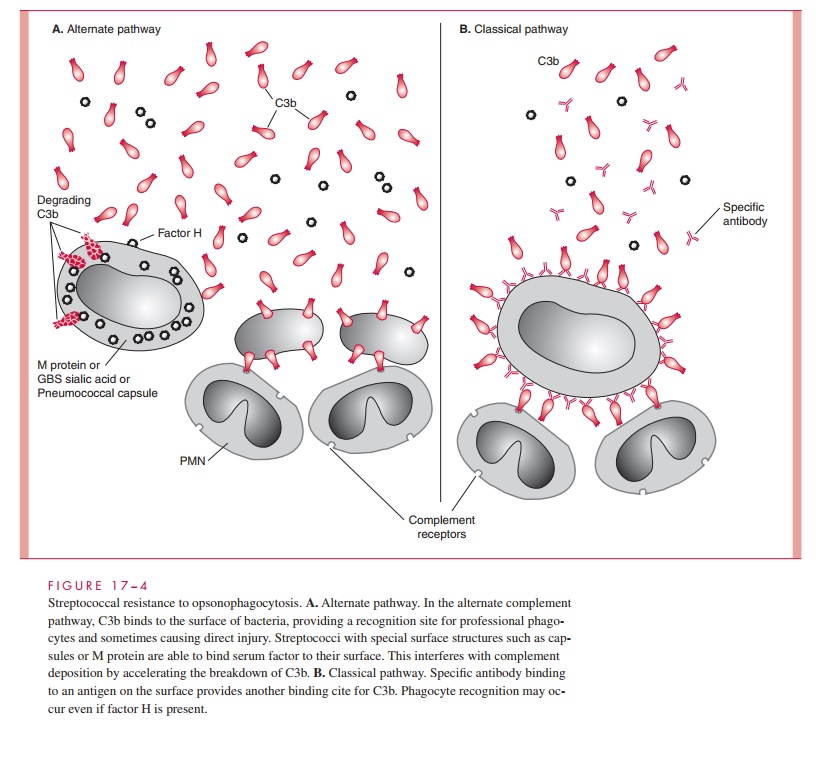
After the initial events of attachment and invasion, it appears that the concerted ac-tivity of the M protein, immunoglobulin-binding proteins, and the C5a peptidase play the key roles in allowing the streptococcal infection to continue. M protein plays an essential role in group A streptococcal resistance to phagocytosis. The antiphagocytic activity of M protein is related to the ability of domains of the molecule to bind fibrinogen and serum factor H. This leads to a diminished availability of alternative pathway generated complement component C3b for deposition on the streptococcal surface (Fig 17 – 4). In the presence of M type – specific antibody, classical pathway op-sonophagocytosis proceeds, and the streptococci are rapidly killed. As a second an-tiphagocytic mechanism the C5a peptidase inactivates C5a and thus blocks chemotaxis of polymorphonuclear neutrophils (PMNs) and other phagocytes to the site of infection. Although the hyaluronic acid capsule contributes to resistance to phagocytosis, the mechanisms involved are unknown.
The precise role of other bacterial factors in the pathogenesis of acute infection is un-certain, but the combined effect of streptokinase, DNAase, and hyaluronidase may prevent effective localization of the infection, while the streptolysins produce tissue injury and are toxic to phagocytic cells. If any of the SPEs are produced, they may contribute as well but their presence is not essential for acute infections. Antibodies against these components are formed in the course of streptococcal infection but are not known to be protective.
In streptococcal toxic shock syndrome (STSS), as with staphylococcal toxic shock syndrome, the findings of shock, renal impairment, and diarrhea seem to be explained by the massive cytokine release stimulated by the superantigenicity of the SPEs. Exotoxin production, however, does not easily explain the enhanced invasiveness of group A strep-tococci, which is an added feature of STSS compared to its staphylococcal counterpart. The enzymatic activity of SPE-B has been linked to invasiveness, but strains with SPE-A and SPE-C are much more common in STSS than SPE-B. This syndrome may represent bacteriophage-mediated horizontal transfer of the SPE genes among recently emerged clones with enhanced invasive potential, a deadly combination. The basis of the enhanced invasiveness remains to be determined.
Poststreptococcal Sequelae
Acute Rheumatic Fever (ARF)
Of the many theories advanced to explain the role of group A streptococci in ARF, an au-toimmune mechanism related to antigenic similarities between streptococci and human tissue antigens has the most experimental support. Streptococcal pharyngitis patients who develop ARF have higher levels of antistreptococcal and autoreactive antibodies and T cells than those who do not. Some of these have been shown to react with both heart tis-sue and streptococcal antigens.
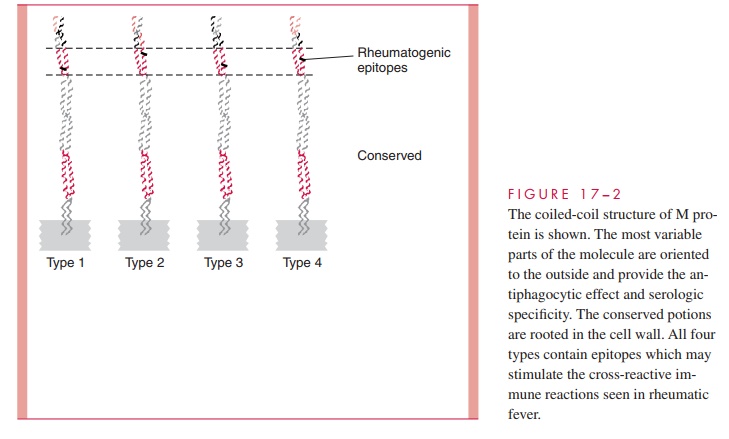
The antigen stimulating these antibodies is most probably M protein, but the group A carbohydrate is also a possibility. The similarity between the structure of M protein and myosin is an obvious connection, and M protein fragments have been shown to stimulate antibodies that bind to human heart sarcolemma membranes. Immunochemical studies of M proteins from different M types are now directed at defining unique epitopes responsi-ble for ARF and the extent to which they are shared between serotypes and strains. Do-mains of the M protein molecule responsible for the heart cross-reactivity have been identified, which differ from those responsible for the factor H and fibrinogen binding. Thus, the cross-reactive and antiphagocytic properties of M protein appear to reside in separate parts of the molecule (see Fig 17 – 2). Antibodies to the dominant epitope of the group A carbohydrate (N-acetylglucosamine) may play a role in injury to the valvular en-dothelium, but T cells stimulated by M protein have been seen in valves as well.
ARF patients also show enhanced cell-mediated immune responses to streptococcal antigens. Cytotoxic T lymphocytes may be stimulated by M protein, and cytotoxic lym-phocytes have been observed in the blood of patients with ARF. A cellular reaction pat-tern consisting of lymphocytes and macrophages aggregated around fibrinoid deposits is found in human hearts. This lesion, called the Aschoff body, is considered characteristic of rheumatic carditis. Suggestions that M protein has superantigen properties must still be reconciled with the prolonged nature of the illness.
Genetic factors are probably also important in ARF because only a small proportion of individuals infected with group A streptococci develop the disease. Attack rates have been highest among those of lower socioeconomic status and vary among those of different racial origins. The gene for an alloantigen found on the surface of B lymphocytes occurs among rheumatic fever patients at a frequency fourfold to fivefold greater than the general population. This further suggests a genetic predisposition to hyperreactivity to streptococcal products.
Acute Glomerulonephritis
The renal injury of acute glomerulonephritis is caused by deposition in the glomerulus of antigen – antibody complexes with complement activation and consequent inflammation. The M proteins of some nephritogenic strains have been shown to share antigenic deter-minants with glomeruli, which suggests an autoimmune mechanism similar to rheumatic fever. Streptokinase has also been implicated both through molecular mimicry and through its plasminogen activation capacity.
IMMUNITY
It has long been known that antibody directed against M protein is protective for subsequent group A streptococcal infections. This protection, however, is only for subsequent infection with strains of the same M type. This is called type-specific immunity. This protective IgG is directed against epitopes in the amino-terminal regions of the molecule and reverses the antiphagocytic effect of M protein. Streptococci opsonized with type-specific antibody bind complement C3b by the classical mechanism, facilitating phagocyte recognition (see Fig 17 – 4). There is evidence that mucosal IgA is also important in blocking adherence while the IgG is able to protect against invasion. Unfortunately, because there are over 80 M types, repeated infections with other M types occurs. Eventually, immunity to the common M types is acquired and infections become less common in adults. In ARF patients, it is the hyperre- action seen in each episode that produces the lesions associated with rheumatic heart disease.
Related Topics