Chapter: Medical Microbiology: An Introduction to Infectious Diseases: Neisseria
Gonorrhea
GONORRHEA
In contrast to meningococcal disease, gonorrhea is primarily localized to mucosal surfaces with relatively infrequent spread to the bloodstream or deep tissues. In- fection is sexually acquired by direct genital contact, and the primary manifesta- tion is pain and purulent discharge at the infected site. In men, this is typically the urethra, and in women, the uterine cervix. Direct extension of the infection up the fallopian tubes produces fever and lower abdominal pain, a syndrome called pelvic inflammatory disease (PID). For women, sterility or ectopic pregnancy can be long-term consequences of gonorrhea.
EPIDEMIOLOGY
Although official reports of gonorrhea in the United States, which represent approxi-mately 50% of the true cases, have been declining for 20 years, the disease is still one of our greatest public health problems. The overall incidence is now 130 cases per 100,000 population, but the rates for adolescents are alarmingly high and increasing by 10% a year. The highest rates are in women between the ages of 15 and 19 years (761/100,000) and men between the ages of 20 and 24 years (564/100,000). No truly effective means of control is yet in sight. Our ability to stem the tide of changed sexual mores continues to be hampered by lack of an effective means to detect asymptomatic cases, resistance of N.gonorrhoeae to antibiotics (seeTreatment), and, to some extent,lackof appreciation ofthe importance of this disease. The latter is evidenced by failure of patients to seekmedical care and of physicians to report cases to public health authorities in order to pro-tect the privacy of their patients. In the minds of too many, syphilis is dreaded and “un-clean,” whereas gonorrhea is only “the clap” (“clap” is from the archaic French clapoir, “a rabbit warren”; later, “a brothel”).
The major reservoir for continued spread of gonorrhea is the asymptomatic patient. Screening programs and case contact studies have shown that almost 50% of infected women are asymptomatic or at least do not have symptoms usually associated with vene-real infection. Most men (95%) have acute symptoms with infection. Many who are not treated become asymptomatic but remain infectious. Asymptomatic male and female patients can remain infectious for months. The attack rates for those engaging in genital in-tercourse with an infected patient are estimated to be 20 to 50%. The organism may also be transmitted by oral – genital contact or by rectal intercourse. When all of these factors operate in a sexually active population, it is easy to explain the high prevalence of gonor-rhea. Although gonococci can survive for brief periods on toilet seats, nonsexual transmis-sion is extremely rare. Fomite transmission of a purulent vulvovaginitis in prepubescent girls has been reported, but virtually all gonococci isolated from children can be traced to sexual abuse by an infected adult.
PATHOGENESIS
Attachment and Invasion
Gonococci are not normal inhabitants of the respiratory or genital flora. When introduced onto a mucosal surface by sexual contact with an infected individual, adherence ligands such as pili, Opa proteins, and possibly LOS allow initial attachment of the bacteria to re-ceptors (CD46, CD66) on nonciliated epithelial cells. Pili are the primary mediators of adherence to urethral and vaginal epithelium, nonciliated fallopian tube cells, sperm, and neutrophils. Opa proteins are involved in cervical and urethral epithelial cell adherence and in adhesion between gonococcal cells.
Following attachment, gonococci invade epithelial cells. The microvilli surround the bacteria and appear to draw them into the host cell in the same manner as meningococci. This process is called parasite-directed endocytosis because it appears to be initiated by bacterial rather than host cell factors and involves cells which are not ordinarily phago-cytic. Gonococcal OMPs such as protein IA and some of the Opa proteins appear to facil-itate this process. Once inside, the bacteria transcytose the cell and exit through the basal membrane to enter the submucosa.
Survival in the Submucosa
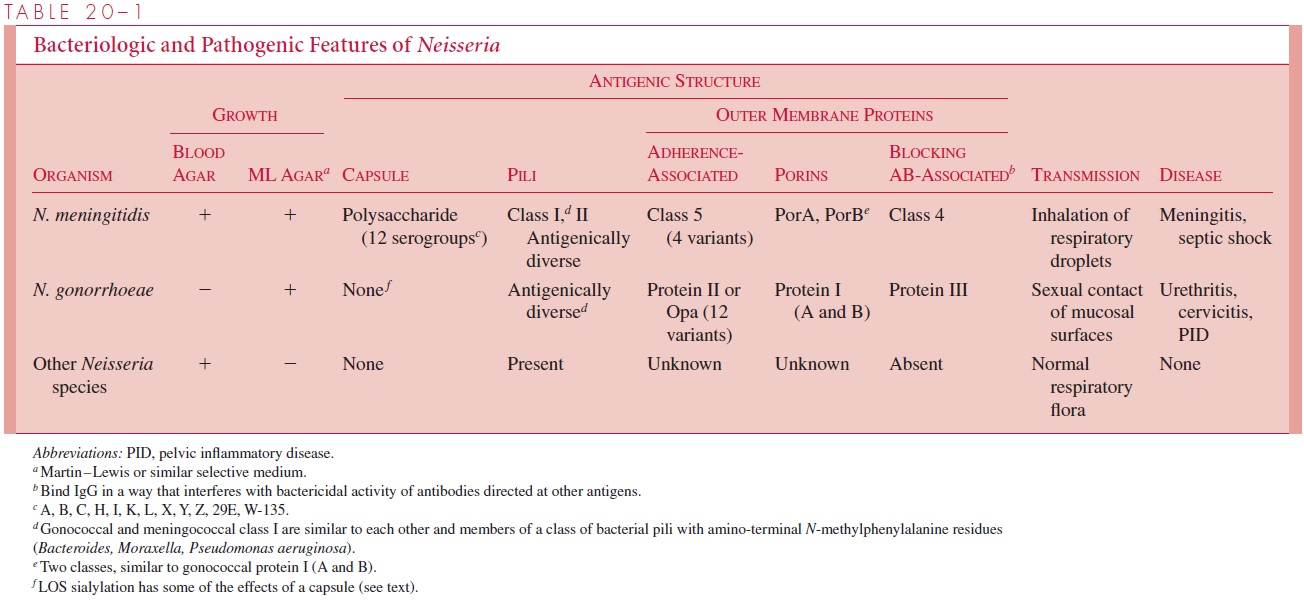
Once in the submucosa, the bacteria must survive and resist innate host defenses as well as defenses that may have been acquired from previous infection. As with meningococci, receptors on the gonococcal surface enable the organisms to scavenge iron needed for growth from the human iron transport proteins transferrin and lactoferrin. Although gono-cocci lack the polysaccharide capsule of the meningococcus, they still have multiple mechanisms that protect them against serum complement and antibody. One of these, LOS sialyation, appears to provide a mechanism for blocking C3b deposition that is iden-tical to that of the encapsulated bacteria. In a sense, the gonococci create their own “capsule” by incorporating host sialic acid into their LOS. Another mechanism for pheno-typic serum resistance is the binding of antibodies to another class of OMPs found in both gonococci and meningococci (see Table 20 – 1). IgG bound to these OMPs appears to block the bactericidal activity of antibodies directed against other surface antigens such as protein I. Blocking antibodies have been found in patients with repeated gonococcal infection.
Even when phagocytes do encounter gonococci, surface factors such as pili and Opa proteins interfere with effective phagocytosis. The organisms are also able to defend against oxidative killing inside the phagocyte by upregulation of catalase production. Taken together, these factors provide ample evidence that killing by neutrophils is sufficiently retarded to allow prolonged survival of gonococci in mucosal and submucosal locations.
Spread and Dissemination
In contrast to meningococci, N. gonorrhoeae bacteria tend to remain localized to geni-tal structures, causing inflammation and local injury, which no doubt facilitate their continued venereal transmission. Purulent exudates containing “sticky” clusters of gonococci held together by Opa proteins could be the primary infectious unit. Infection may spread to deeper structures by progressive extension to adjacent mucosal and glan-dular epithelial cells. These include the prostate and epididymis in men and the para-cervical glands and the fallopian tubes in women. Spread to the fallopian tubes may be facilitated by pilus-mediated attachment to sperm and then to the microvilli of noncili-ated fallopian tube cells. Injury to the fallopian epithelium seems to be mediated by LPS/LOS and fragments of gonococcal cell wall peptidoglycan. Gonococci are known to turn over their peptidoglycan rapidly during exponential growth, releasing peptido-glycan fragments into the local environment. Injury by this mechanism has been demonstrated in fallopian tube organ cultures and presumably may also operate at other sites.
In a small proportion of infection, organisms reach the bloodstream to produce dis-seminated gonococcal infection (DGI). When this happens, the systemic findings have their own pattern (see Manifestations) and seldom take on the endotoxic shock picture of meningococcemia. Although differences have been noted between N. gonorrhoeae strains that remain localized and those that produce DGI, their connection to pathogenesis is un-known. Both DGI and salpingitis tend to begin during or shortly after completion of menses. This may relate to changes in the cervical mucus and reflux into the fallopian tubes during menses.
Genetic Regulation of Virulence
Through all the stages of gonorrhea, gonococci are able to use a particularly rich variety of genetic mechanisms in deployment of the virulence factors described above at the right time. Some are regulatory responses to environmental cues, such as iron in relation to iron-binding proteins, while others involve the changes in the genome. Antigenic changes in both pili and Opa proteins have been demonstrated in human infection, including the isola-tion of antigenic variants from different sites in the same patient. These presumably take place by the recombinational and translational mechanisms (see Antigenic Variation) as the organisms replicate in the patient.
IMMUNITY
The apparent lack of immunity to gonococcal infection has long been a mystery. Among sexually active persons with multiple partners, repeated infections are the rule rather than the exception. Both serum and secretory antibodies are generated during natural infection but the levels are generally low, even after repeated infections. An-other aspect is that even when antibodies are formed, antigenic variation defeats their effectiveness and allows the gonococcus to escape immune surveillance. Antigenic variation of pili, Opa proteins, and LOS is particularly likely to be important. Out-breaks have been traced to a single strain that demonstrated multiple pilin variations and Opa types in repeated isolates from the same individual or from sexual partners. In experimental models, passive administration of antibody directed against one pilin type has been followed by emergence of new pilin variants. Changes in Opa proteins may also occur, as suggested by differences in its expression in mucosal versus tubal iso-lates. It appears that although some immunity to gonococcal infection is present, its effectiveness is compromised by the ability of the organism to change key structures during the course of infection.
Related Topics