Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
General characteristics of p-block elements
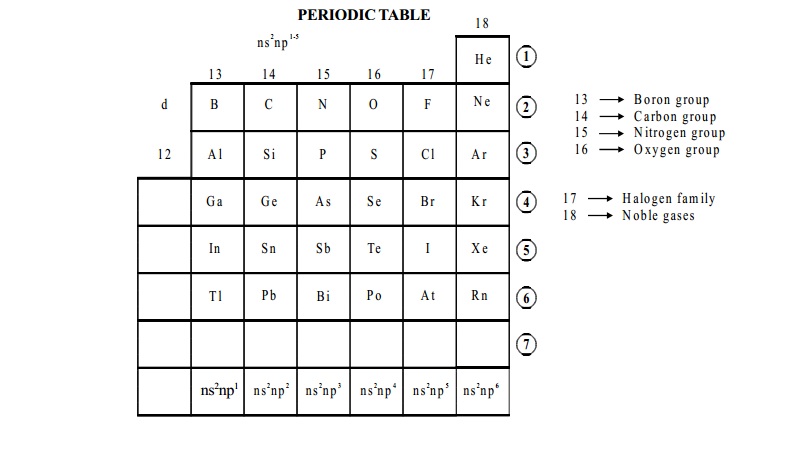
13 - Boron group
14 - Carbon group
15 - Nitrogen group
16 - Oxygen group
17 - Halogen family
18 - Noble gases
p-block elements grouped with s-block elements are
called as main group elements or
representative elements. There are 44 main group elements. p-block elements occupy groups 13-18 of the periodic table
including inert gases. p-block elements play
dominant part in all natural processes. Aluminium plays vital role in aircraft and as conductors. Carbon is the
backbone of all organic compounds. Silicon
chips play a vital part in computers. Nitrogen acts as a building block of life. Molecular oxygen is a cell fuel.
General characteristics of p-block elements
1.
The general
electronic configuration of p-block elements is ns2 np1-6.
2.
These elements
include metals and non-metals with a few semi metals (Metalloids)
3.
Most of them form
covalent compounds.
4.
These elements
possess relatively higher ionisation energy and the value tends to increase along the period but decrease down the
group.
5.
Most of the
elements show negative (except some metals) as well as positive oxidation states (except Fluorine).
6.
One of the familiar
characteristic of p-block elements is to show inert pair effect i.e. the tendency of being less availability for
ns electron in bonding. The inert pair
effect increases down the group with the increase in atomic number.
Group 13 elements - The Boron family
The group 13(IIIA) elements are Boron, aluminium,
gallium, indium and
thallium.
1.
Boron is a
relatively rare element, accounting for only about 0.001% of the earth's crust by mass.
2.
Aluminium is the
most important of 13th group elements.
3.
Gallium is
remarkable for its unusually low melting point (29.7 o C) and therefore generally exist as a liquid at room
temperature. Its most important use
is in making gallium arsenide. This is a semi conductor material employed in the manufacture of diode lasers for laser printers,
compact -disc players and fibre optic
communication devices.
4.
Indium is also used
in making semi conductor devices, such as transistors and electrical resistance thermometers called
thermistors.
5.
Thallium is
extremely toxic and has no commercial use.
Related Topics