Chapter: Modern Pharmacology with Clinical Applications: Gene Therapy
Gene Addition
GENE ADDITION
A more practical approach has
been to permit the in-troduced genes to integrate into the genome in a
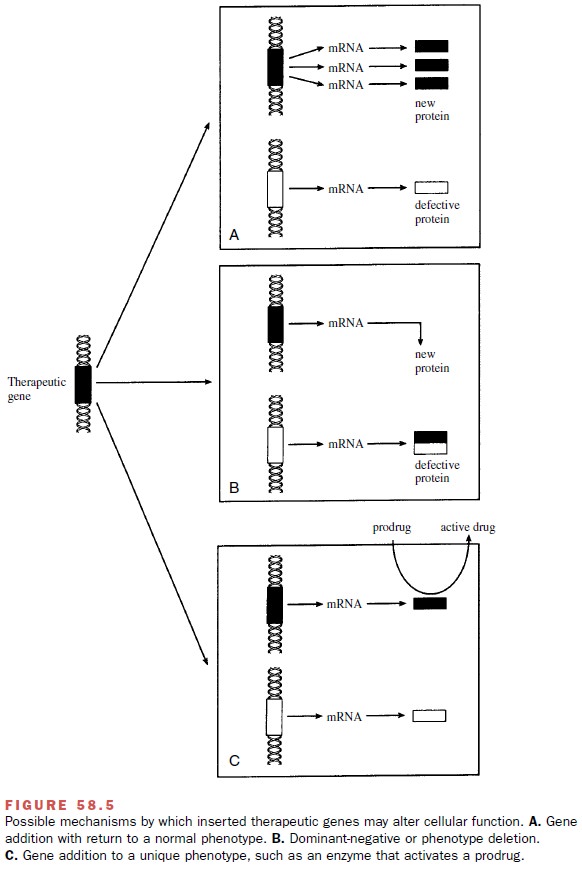
site-nonspecific manner. The newly added gene could then function to provide a
missing or mutated gene product (Fig. 58.5A).
This is the approach of most current gene
therapy protocols and is exemplified by
the development of clinical trials for adenosine deaminase (ADA) defi-
ADA is a reasonable target for these reasons: (1) It is an auto-somal
recessive disorder in which a defect in a single gene produces absence of or
diminished ADA activity with fatal combined immunodeficiency. (2) ADA
ex-pression is characteristic of a normal maintenance gene with considerable
variation in the normal ADA levels, suggesting that stringent regulation of
expression is un-necessary. (3) A significant level of expression is not re-quired
to correct the phenotype. (4) Ex vivo gene trans-fer studies can be conducted.
(5) Replacement of ADA may reduce the production of toxic DNA metabolites and
thus provide a growth advantage for transfected cells.
For ethical reasons, children
enrolled in these clini-cal trials have also received standard therapy of
enzyme infusions, so the results of these studies have been diffi-cult to
interpret and are controversial. Nevertheless, there is some evidence that the
ex vivo gene transfer ap-proach may evoke a biological response relevant to the
treatment of ADA deficiency. Such interpretations have stimulated efforts to
use the ex vivo strategy for other monogenic disorders, such as familial
hypercholes-terolemia, hemophilia B, and Gaucher’s disease.
Alternatively, the introduced
gene could generate a protein that acts to block or suppress the function of
an-other undesirable protein in a dominant-negative man-ner (Fig. 58.5B). Last, the introduced gene could
result in the production of an entirely new and unique protein that provides
the recipient cell with a desirable pheno-type (Fig. 58.5C). In theory, an enzyme required for the metabolic activation of a
prodrug could be expressed, leading to the desired pharmacological activity
near the genetically altered cell. This approach is used in cancer gene therapy
in which tumor cells are transfected with a gene encoding for an enzyme such as
thymidine kinase
The transfected enzyme in the tumor cells
con-verts the prodrug, such as ganciclovir, to an active cyto-toxic compound.
Theoretically, such an approach selec-tively kills tumor cells and is nontoxic
to untransfected cells. Clinical trials to assess the safety and efficacy of
enzyme–prodrug cancer therapy are under way.
Related Topics