Chapter: 11th Chemistry : UNIT 12 : Basic concepts of organic reactions
Fission of a covalent bond
Fission
of a covalent bond
All
organic molecules contain covalent bonds which are formed by the mutual sharing
of electrons between atoms. These covalent bonds break in two different ways,
namely homolytic cleavage (symmetrical splitting) and heterolytic cleavage
(unsymmetrical splitting). The cleavage of a bond in the substrate is
influenced by the nature of the reagent (attacking agent).
Homolytic Cleavage
Homolytic
cleavage is the process in which a covalent bond breaks symmetrically in such
way that each of the bonded atoms retains one electron. It is denoted by a half
headed arrow (fish hook arrow). This type of cleavage occurs under high
temperature or in the presence of UV light in a compound containing non polar
covalent bond formed between atoms of similar electronegativity. In such
molecules, the cleavage of bonds results into free radicals. They are short lived
and are highly reactive. The type of reagents that promote holmolytic cleavage
in substrate are called as free radical initiators. For example
Azobisisobutyronitile (AIBN) and peroxides such as benzoyl peroxide are used as
free radical initiators in polymerisation reactions.
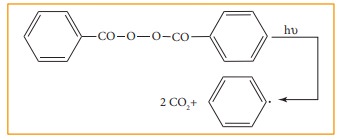
As
a free radical with an unpaired electron is neutral and unstable, it has a
tendency to gain an electron to attain stability. Organic reactions involve
homolytic fission of C- C bonds to form alkyl free radicals. The stability of
alkyl free radicals is in the following order
˙C(CH3)3 > ˙CH(CH3)2>˙CH2CH3 >˙CH3
Heterolytic Cleavage
Heterolytic
cleavage is the process in which a covalent bond breaks unsymmetrically such
that one of the bonded atoms retains the bond pair of electrons. It results in
the formation of a cation and an anion. Of the two bonded atoms, the most
electronegative atom becomes the anion and the other atom becomes the cation.
The cleavage is denoted by a curved arrow pointing towards the more
electronegative atom.
For
example, in tert-butyl bromide, the C-Br bond is polar as bromine is more
electronegative than carbon. The bonding electrons of the C-Br bond are
attracted more by bromine than carbon. Hence, the C-Br undergoes heterolytic cleavage
to form a tert-butyl cation during hydrolysis.
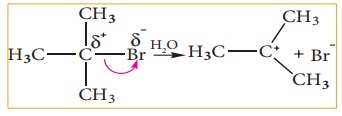
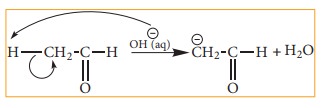
Let
us consider the cleavage in a carbon-hydrogen (C-H) bond of aldehydes or
ketones We know that the carbon is more electronegative than hydrogen and hence
the heterolytic cleavage of C-H bonds results in the formation of carbanion
(carbon bears a negative charge). For example in aldol condensation the OH- ion
abstracts a α-hydrogen from the aldehyde, which leads to the formation of the
below mentioned carbanion.
Hybridisation of carbon in carbocation:
In
a carbocation, the carbon bearing positive charge is sp2 hybridised and hence
it has a planar structure. In the reaction involving such a carbocation, the
attack of a negatively charged species (nucleophiles) take place on either side
of the carbocation as shown below.
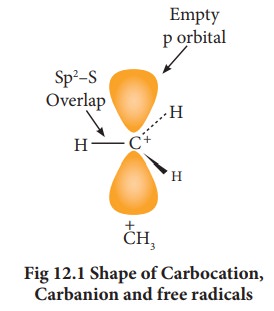
The
carbanions are generally pyramidal in shape and the lone pair occupies one of
the sp3 hybridised orbitals. An alkyl free radical may be either
pyramidal or planar.
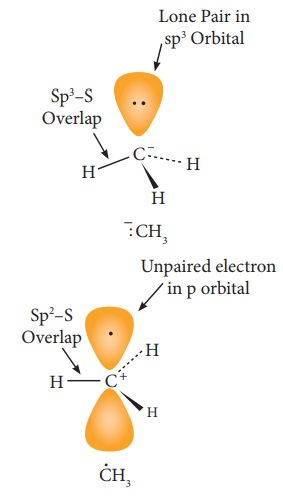
The
relative stability of the alkyl carbocations and carbanions are given below.
Relative
stability carbocations.
+C(CH3)3 > +CH(CH3)2
> +CH2CH3
> +CH3
relative
stability of carbanions
–C(CH3)3 < –CH(CH3)2
< –CH2CH3
< –CH3
The energy required to bring about homolytic splitting is greater than that of heterolytic splitting.
Related Topics