Chapter: The Diversity of Fishes: Biology, Evolution, and Ecology: Chondrichthyes: sharks, skates, rays, and chimaeras
Feeding habits - Subclass Elasmobranchii
Feeding habits
Sharks are apex predators throughout the world, stationed at the top of the food webs in which they occur. All elasmobranchs are carnivorous, taking live prey or scavenging on recently dead animals. No evidence of herbivory or detritivory exists, and the only departures from feeding on relatively large prey are the huge, filter-feeding, zooplanktivorous Basking, Megamouth, and Whale sharks and manta rays. For most shark species, bony fishes constitute 70–80% of the diet. Feeders on other prey types include carcharhinid Tiger, Bull, and Galápagos sharks (other sharks); hammerheads (stingrays); White, Tiger, Sleeper, and Cookie Cutter sharks (marine mammals); Tiger Sharks (seabirds); and Leopard, Nurse, and Green Dogfish sharks and most skates and rays (invertebrates). Somniosid sleeper sharks in Antarctica feed on the ocean’s largest invertebrates, the giant and colossal squids (Cherel & Duhamel 2004).
The characteristic ventral mouth of most sharks (exceptions include the Megamouth, Frill, Whale, and angel sharks and the manta rays) is apparently linked to dependence on bite strength rather than suction during feeding. Nurse sharks, as well as some heterodontiform sharks and many rajiforms, utilize suction extensively while feeding, but negative pressures are produced by expansion of the enlarged orobranchial cavity rather than enlargement of the mouth via jaw protrusion. Underslung jaws in sharks provide larger regions for muscle attachment than in fishes whose jaw bones extend all the way to the tips of their snouts. The shark confi guration allows for both protrusion of the palatoquadrate (upper jaw) and the generation of the powerful biting forces required to cut through the skin, bone, and muscle of their prey (Fig. 12.8). Hence sharks are not limited to prey that can be swallowed whole, whereas the vast majority of osteichthyans are “gapelimited” and risk suffocation if they attack prey larger than they can swallow whole.
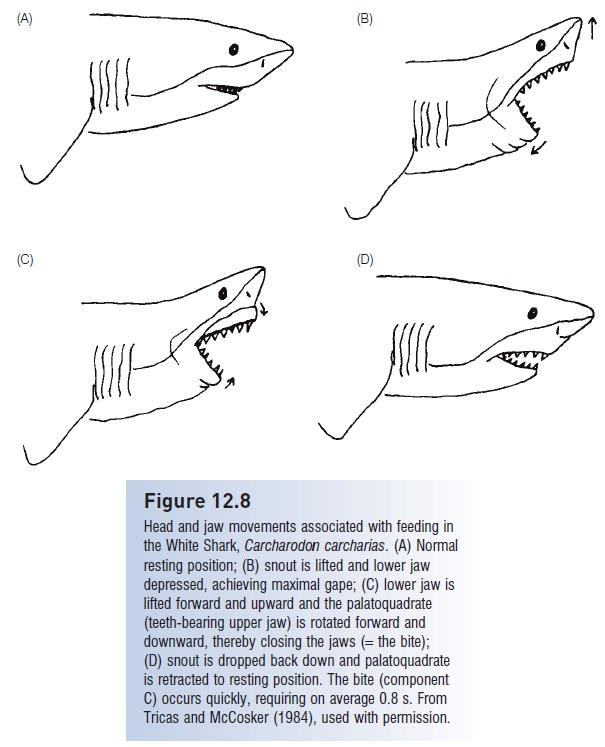
Figure 12.8
Head and jaw movements associated with feeding in the White Shark, Carcharodon carcharias. (A) Normal resting position; (B) snout is lifted and lower jaw depressed, achieving maximal gape; (C) lower jaw is lifted forward and upward and the palatoquadrate (teeth-bearing upper jaw) is rotated forward and downward, thereby closing the jaws (=the bite); (D) snout is dropped back down and palatoquadrate is retracted to resting position. The bite (component C) occurs quickly, requiring on average 0.8 s. From Tricas and McCosker (1984), used with permission
Separation of the palatoquadrate from the cranium serves as more than a diagnostic character for identifying elasmobranchs. This separation also allows upper jaw teeth to be retracted during nonfeeding periods, which aids in streamlining of the head profile.Protrusion during the bite
increases bite efficiency, jaw closure speed, and prey manipulation (Wilga et al. 2001). Hence prey can be efficiently cut by the closing action of the jaws and serrate teeth, rather than merely grasped.
In many carcharhiniform and lamniform sharks, lower jaw teeth are spikelike whereas upper jaw teeth are flatbladed with serrated edges. In a typical jaw closing sequence (Fig. 12.8), the lower jaw is lifted first, impaling the prey, and then the upper jaw is brought down, once or repeatedly. Once prey are grasped, many sharks also shake their head and upper body, or even rotate the entire body about its long axis. During this movement, the upper jaw teeth slice through the prey, eventually removing a chunk of flesh (Moss 1977, 1984; Tricas & McCosker 1984). Jaw protrusion is extreme in some rajiforms due to the loss of ligamental connections between the palatoquadrate and cranium. These fishes dexterously pick up food objects from the bottom with their jaws through a combination of suction and grasping (Moss 1977, 1984).

Figure 12.9
Cookie cutter sharks. (A) Isistius brasiliensis, the Cookie Cutter Shark, is a small (about 40 cm) tropical species that lives at mid-ocean depths and parasitizes tunas, other fishes, Megamouth Sharks, and marine mammals, gouging circular plugs of flesh out of their sides with its specialized dentition. (B) The congeneric Largetooth Cookie Cutter Shark, I. plutodus, has the largest teeth for its body size of any known shark. Its teeth are twice as large relative to body size as a Great White Shark’s teeth. (C) Drawing of the Cookie Cutter Shark, I. brasiliensis. (A) photo by C. S. Johnson, from Springer and Gold (1989), used with permission of the Smithsonian Institution Press; (B) from Compagno (1981), used with kind permission from Kluwer Academic Publishers; (C) after P. Vecsei.
One departure from this norm occurs in some squaloid sharks, in which the lower teeth are more bladelike than the uppers and are arranged in a flat band across the lower jaw, perpendicular to the axis of the body. To this group belongs the highly specialized, 40 cm long, bioluminescent Cookie Cutter Shark, Isistius brasiliensis (Dalatiidae), the only ectoparasitic elasmobranch (Fig. 12.9). Craterlike wounds about 4 cm across are frequently found on tunas, dolphins, whales, and even Megamouth Sharks, all animals that spend part of their time at mesopelagic depths (200– 1000 m). These wounds match precisely the mouth size of the Cookie Cutter Shark and it has been deduced that the small sharks lurk among small mesopelagic organisms and prey on the larger predators that move into this region to feed (Jones 1971; Shirai & Nakaya 1992). The pattern and nature of the green-emitting photophores (light organs) on Isistius suggest that it is essentially invisible because its light output closely matches the spectrum of background light at the depths where it swims (Widder 1998). The symmetrical craterlike wounds may be formed when the shark attaches to the side of its victim and then spins about its long axis, removing a flat cone of tissue (E. Clark, pers. comm.). Similar but much larger wounds on the sides of seals and beluga whales may be the result of attacks by large Greenland Sharks (Somniosidae), which have a dentition pattern similar to that of Isistius (A. Fisk, pers. comm.).
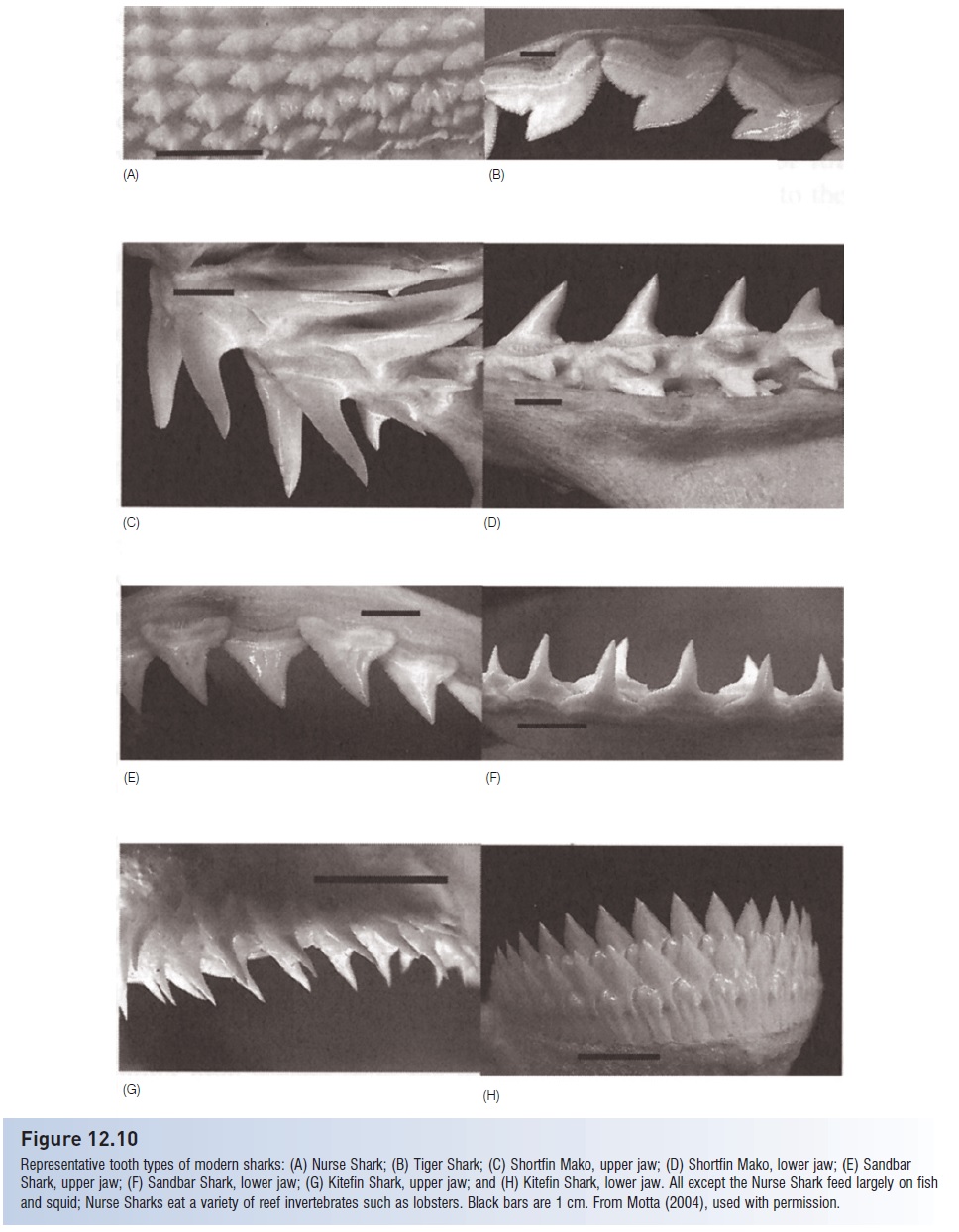
Figure 12.10
Representative tooth types of modern sharks: (A) Nurse Shark; (B) Tiger Shark; (C) Shortfin Mako, upper jaw; (D) Shortfin Mako, lower jaw; (E) Sandbar Shark, upper jaw; (F) Sandbar Shark, lower jaw; (G) Kitefin Shark, upper jaw; and (H) Kitefin Shark, lower jaw. All except the Nurse Shark feed largely on fish and squid; Nurse Sharks eat a variety of reef invertebrates such as lobsters. Black bars are 1 cm. From Motta (2004), used with permission.
Dentition morphology, an important taxonomic characteristic for identifying species, correlates strongly with food type in most sharks (Fig. 12.10). Predators on fishes and squids, such as mako, Sand Tiger, and Angel sharks, have long, thin, piercing teeth for grasping whole prey, which they often swallow whole. Most requiem sharks, which are also piscivores, have such piercing teeth in the lower jaw and bladed teeth with finely serrate edges for cutting prey in the upper jaw (see above). Sharks and rays that feed on hard-shelled prey such as mollusks and large crustaceans have specialized, broad dentition for crushing (Fig. 12.11).
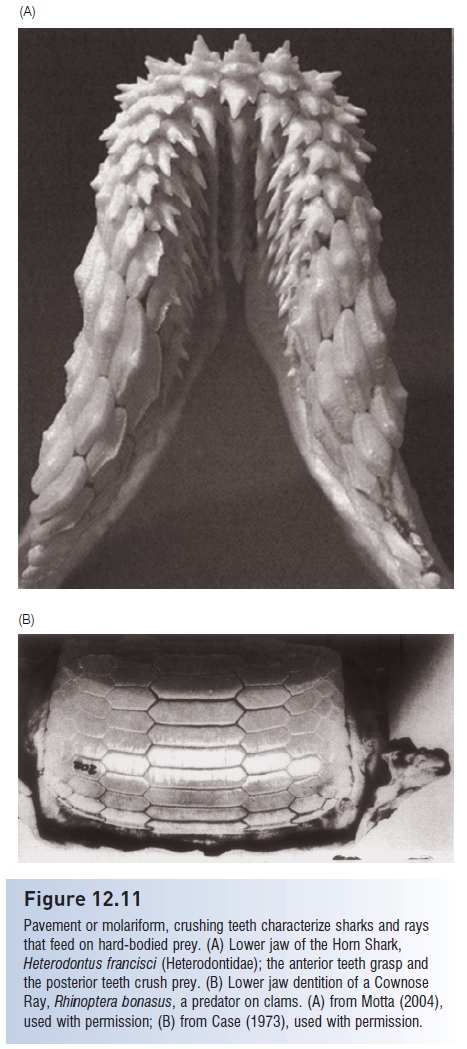
Figure 12.11
Pavement or molariform, crushing teeth characterize sharks and rays that feed on hard-bodied prey. (A) Lower jaw of the Horn Shark,
Heterodontus francisci (Heterodontidae); the anterior teeth grasp and the posterior teeth crush prey. (B) Lower jaw dentition of a Cownose Ray, Rhinoptera bonasus, a predator on clams. (A) from Motta (2004), used with permission; (B) from Case (1973), used with permission
(Bamboo Sharks can feed on hard-bodied prey with sharp teeth by bending the teeth backwards such that the anterior surfaces crush rather than pierce the prey, Dentition.)
Whereas most sharks engage only the anterior, peripheral row or rows of teeth while feeding, in mollusk feeders most of the posterior rows participate in the crushing action. Filter-feeding sharks typically have greatly reduced teeth that may function minimally during feeding; they instead use their gill rakers to trap small prey. In bony fishes, teeth are attached directly to the bone of the jaws, whereas in sharks the teeth are embedded in the connective tissue. Sharks have teeth only on their jaw margins, not attached to other bones and structures in the mouth, as in bony fishes. Shark teeth are basically enlarged scales, derived embryologically from the same tissues as the dermal denticles.
Dentition replacement is characteristic of all sharks, although detailed information on patterns of replacement is limited to a few species. Some sharks, such as White Sharks and hammerheads, replace teeth individually as they are worn out or lost. In contrast, Spiny Dogfish, Greenland, and Cookie Cutter sharks apparently replace entire rows. Replacement occurs regardless of use; nonfunctional teeth in interior rows continue to grow and move forward, eventually displacing or replacing functional teeth. Teeth also grow as the shark grows and hence teeth in the most internal, nonfunctional rows are larger than the functional teeth about to be replaced. Turnover rates have been calculated for a few species in captivity. Lemon Sharks replace a functional tooth about every 8–10 days, Sand Tigers every 2 days, Leopard Sharks every 9–12 days, Nurse Sharks every 9–28 days, and Horn Sharks every 28 days (Motta 2004). Given these numbers, it has been estimated that a shark may produce on the order of 30,000 teeth during its lifetime (Moss 1967; Springer & Gold 1989; Overstrom 1991).
Some sharks use structures other than jaw teeth to capture prey. Thresher sharks possess a long, scythelike upper caudal lobe. Threshers herd fish into tight schools by circling and splashing with the tail, then stunning prey with quick whips of the tail. Evidence that thresher sharks use their tails in prey capture comes from observations of many threshers caught by the tail on baited hooks (Compagno 1984).
Two unrelated families of elasmobranchs have evolvedarmed rostral regions used in prey acquisition. Pristid sawfishes (Rajiformes) and pristiophorid sawsharks (Pristiophoriformes) both possess bladelike snouts armed with lateral teeth (modified denticles) which they slash laterally to stun and disable prey. The lateral projections of the rostral cartilage of hammerhead sharks have long intrigued anatomists. Recent observations by divers indicate thathammerheads, which tend to specialize on stingrays, may use the hammer to pin their stingray prey to the bottom while taking bites from the margins of the stingray’s disk.
A spectacular non-oral prey capture device is evident in the strong electric discharges of torpedo rays (Torpedinidae). These batoids possess modified hyoid and branchial musculature capable of emitting electric discharges of up to 50 volts and 50 amps, producing an output approaching 1 kW.
Although electric discharges can occur when the torpedo is disturbed and hence may serve as a predator deterrent, the more interesting function appears to be in prey capture. Torpedos will lie on the bottom and ambush prey during the daytime, but at night they swim or drift slowly in the water column. Upon encountering a potential prey fish, they envelope it with their pectoral fins and emit pulsed electric outputs that are modified in terms of rate and duration in response to prey reaction. The stunned, immobilized prey fish is then pushed towards the torpedo’s mouth via water currents produced by undulations of the pectoral disk (Bray & Hixon 1978; Fox et al. 1985; Lowe et al. 1994). Torpedos differ from most other batoids in that they possess a well-developed caudal fin that is used during locomotion. Most batoids use their pectoral fins for locomotion, but this anatomical region has been usurped for electric organ function in torpediniforms.
Filter feeding occurs in four different groups of sharks and rays and in each has taken a slightly different evolutionary route. The Megamouth Shark, Megachasma pelagios (Megachasmidae), is the most spectacular shark species discovered in the 20th century (Fig. 12.12). MegamouthSharks feed on euphausiid krill, jellyfish, and small schooling fishes. Megamouth cartilage is poorly calcified and body musculature is relatively soft, suggesting a sluggish filterfeeder with a terminal mouth. Prey are ingested via suction and then captured on dense gill raker papillae (Compagno 1990c; Yano et al. 1997).
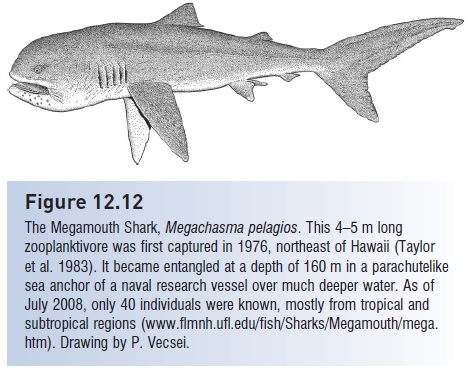
Figure 12.12
The Megamouth Shark, Megachasma pelagios. This 4–5 m long zooplanktivore was first captured in 1976, northeast of Hawaii (Taylor et al. 1983). It became entangled at a depth of 160 m in a parachutelike sea anchor of a naval research vessel over much deeper water. As of July 2008, only 40 individuals were known, mostly from tropical and subtropical regions www.flmnh.ufl.edu/fish/Sharks/Megamouth/mega. htm). Drawing by P. Vecsei.
Manta rays, which can be over 6 m wide, filter feed via ram feeding, which involves swimming with the mouth open. Small crustaceans and fishes are additionally guided into the pharynx with the cephalic horns and the prey are then caught on pharyngeal filter ridges. The cephalic horns are anterior subdivisions of the pectoral fins, making manta rays the only extant vertebrates with three functioning pairs of limbs. Whale Sharks use ram feeding while swimming and also a suction/gulp-and-drain mechanism when stationary to feed on plankton or small fish, which are filtered through cartilaginous rods (Compagno 1984, 1990c). Basking Sharks swim at a constant speed of about 1 m/s with their mouths open, using passive ram filtration that employs gill raker denticles and mucous to capture very small zooplankters. Basking Sharks were thought to shed raker denticles, cease feeding, and enter a torpid state when zooplankton abundances declined in winter. However, recent behavioral observations, plankton density measurements, tracking with archival tags, and energetics calculations indicate that most retain their denticles, can feed well into the winter, and migrate to regions of high plankton productivity during colder months and continue feeding (Sims 1999; Sims et al. 2003).
Although sharks swim actively throughout the diel cycle and will capitalize on opportunities to feed at any time, available data indicate that the primary foraging times of most species are during twilight and nighttime. Such crepuscular/ nocturnal foraging has been confi rmed by catch statistics and more recently by ultrasonic telemetry and direct observation. Catches of sharks on baited hooks are greater by night than by day. Activity cycle data from telemetered Lemon Sharks indicate a doubling of swimming speed during twilight as compared to daytime or nighttime speeds (Sundstrom et al. 2001). Increased nocturnal swimming has also been found in Gray Reef, Blue, Scalloped Hammerhead, Horn, angel, and Swell sharks, as well as torpedo rays (Myrberg & Nelson 1991). In contrast, the White Shark is primarily a diurnal feeder, at least where its major prey is diurnally active marine mammals (Klimley et al. 1992; Klimley 1993).
Related Topics