Chapter: Surgical Pathology Dissection : The Ocular System
Eye : Surgical Pathology Dissection
Eye
General Comments
The eye
is a unique neurosensory organ. Much information can be gathered from the gross
and microscopic examination of enucleated globes regarding the pathogenesis and
manifestations of ocular and systemic diseases.
Enucleation Specimens
Fixation
The most
commonly used fixative for ocular tis-sues is 10% neutral buffered formalin (4%
for-maldehyde), but even this standard fixative has its disadvantages. The
relatively high osmolarity of 10% neutral buffered formalin may cause
con-traction of the anterior chamber and vitreous cavity, which may result in
artifactual detach-ment of the retina. Moreover, formalin tends to dissolve
water-soluble substances such as glyco-gen, resulting in shrinkage of fixed
tissue. Thus, it is even a less suitable fixative when electron microscopy
studies are required. The amount of 10% neutral buffered formalin required for
ade-quate fixation varies with the size of the ocular specimen. For example, a
small specimen such as a cornea or lid biopsy may require only 5 to 10 ml of formalin;
an enucleated globe, 150 to 300 ml of formalin; and an orbital exenteration,
500 ml of formalin.
One
alternative to formalin is glutaraldehyde 4%. This solution provides adequate
fixation forboth light and electron microscopy, while causing much less tissue
shrinkage because of its lower osmolarity. For specimens less than 2 mm in
di-ameter, glutaraldehyde 2.0% to 2.5% may be the preferred primary fixative
for electron micros-copy. Glutaraldehyde, however, causes tissues to become
hard and brittle and may adversely affect the staining of tissues. For example,
ocular tissues fixed in glutaraldehyde 4% stain less vividly with alcian blue
and colloidal iron tech-niques, and they stain diffusely and nonspecifi-cally
with the periodic acid-Schiff (PAS) reaction. Yet another alternative is to
combine fixatives to achieve optimal preservation for both light and electron
microscopy. For example, a solution that combines 4% formaldehyde and 1%
gluta-raldehyde in phosphate buffer 0.1 mol/L can be used.
Fixation
of ocular tissues requires patience. Practices that are designed to speed up
the pro-cess (e.g., opening the globe, cutting windows into the sclera, or
injecting fixative directly into the vitreous) are strongly discouraged because
they are likely to induce artifactual disruption of the ocular tissues. The
fixation of an enucleated globe in formalin generally requires 24 to 48 hours.
After fixation, gently rinse the globe in running water for 16 hours, then
place it in ethyl alcohol 60% for grossing.
Eyes
that contain excessive calcium deposits or even bone formation may require
special decalci-fying agents in addition to routine fixatives. In these cases,
first fix the globe and then decalcify it in a solution of sodium citrate and
formic acid for 24 to 72 hours. When the specimen is soft enough to section,
wash it overnight in running tap water to remove all traces of acid, and then
place the specimen in ethyl alcohol 60% before further processing. Because
decalcification ob-scures histologic detail and interferes with staining, check
the specimen daily while it is in solution to avoid excessive decalcification.
External Examination
Proper
orientation of the eye is essential to docu-ment the location of a lesion
within the eye. Although the eye is roughly spherical, careful attention to
external landmarks allows one to orient this structure with respect to the
horizontal median and nasal aspect. One such landmark is the cornea. The cornea
occupies the anterior one sixth of the globe, measuring about 11 mm in its
vertical plane and 11.5 mm in its horizontal plane. Thus, the longer axis of
the cornea indicates the horizontal meridian. Other external land-marks are
even more helpful in orienting the eye. The posterior ciliary arteries, for
example, can be used to determine both the horizontal plane and the nasal
aspect of the specimen. These ves-sels enter the sclera in the region of the
optic nerve and then extend horizontally. Importantly, the nasal vessel is
usually more prominent and therefore can be used to identify the nasal aspect
of the eye. The nasal aspect can also be identified by measuring the distance
between the limbus (the periphery of the cornea where it joins the sclera) and
the optic nerve. This distance is short-est along the nasal aspect.
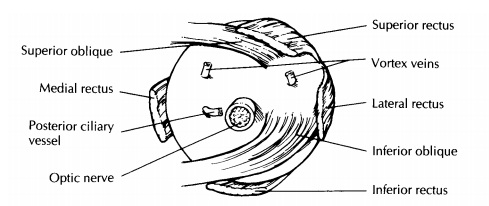
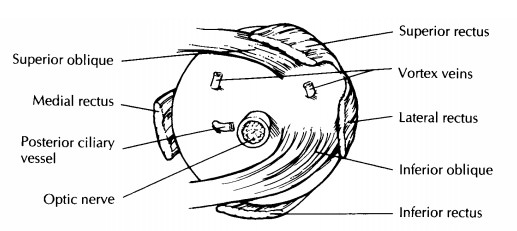
Perhaps
the most reliable landmarks for ori-enting the eye are the insertions of the
extraocular muscles. The use of these landmarks, however, requires a good
understanding of ocular anatomy (Fig. 35-1). The tendon of the superior oblique
muscle extends temporally from the trochlea in the nasal orbital wall to insert
into the sclera su-perotemporally posterior and just temporal to the superior
rectus insertion and superior to the optic nerve. The inferior oblique muscle
extends tem-porally from the inferonasal orbital wall to insert into the sclera
(as a muscular rather than as a tendinous insertion) just temporal to the optic
nerve and posterior ciliary vessel. The insertion of the inferior oblique
muscle overlies an area of the sclera corresponding to the macula inside the
eye.
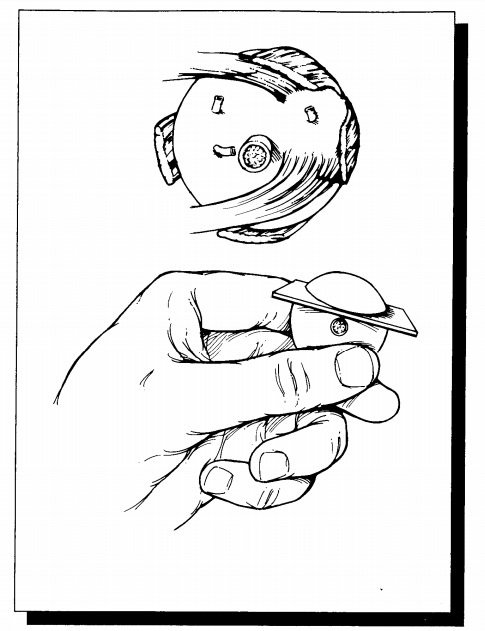
Once the
eye has been properly oriented, mea-sure its anteroposterior, horizontal, and
vertical dimensions. Record all measurements in millime-ters. Also record the
dimensions of the cornea and the length of the optic nerve stump. Describeany
abnormal external features such as corneal opacities and lacerations or wounds
of the cornea and/or sclera. A careful external examination will often disclose
important information regarding a history of eye pathology. Scars located at
the superior limbus suggest prior surgery for cata-racts or glaucoma, and the
presence of a silicone band or sponge suggests prior surgery for retinal
detachment. If these silicone bands and sponges are encountered, they need not
be dislodged, be-cause they will dissolve during processing. On the other hand,
metal clips should be meticu-lously removed from any area submitted for
his-tologic evaluation.
For
cases of suspected melanoma, carefully ex-amine the outer surface of the
specimen for tumor spread. Specifically, examine the vortex veins for
engorgement by tumor, check the episcleral soft tissues for pigment deposition,
and look for gross extrabulbar extension by the tumor. In cases of suspected
retinoblastoma, carefully examine the optic nerve grossly, and take the
surgical margin of the optic nerve for microscopic exami-nation. A dissecting
microscope is extremely helpful in identifying minute lesions, and it should be
employed during the external and in-traocular examinations.
Photography
plays an integral part of the gross examination of ocular tissues. As discussed
in earily, photographs are useful for document-ing any abnormal features of the
external globe and are very helpful in correlating these gross features with
the clinical findings. Likewise, pho-tographs should be taken of intraocular
lesions after the eye has been opened. The best photo-graphs are obtained with
the specimen submerged in alcohol (60%) and with even illumination.
Transillumination
of the globe plays an im-portant role in the localization of intraocular tumors
that cannot be directly visualized on external examination. To transilluminate
the specimen, place the eye in front of a small in-tense light against a dark
background. One method is to use a substage microscope lamp in a dark room. Rotate
the globe over the light source, and look for areas of increased or de-creased
transmission of light. Increased trans-mission of light may be seen in defects
of the iris as occur in pigmentary dispersion syn-drome and following
peripheral iridectomy or cataract surgery. Decreased transmission of light may
be due to intraocular hemorrhage or intraocular tumors. Mark these
transillumination
Transillumination
of the globe plays an im-portant role in the localization of intraocular tumors
that cannot be directly visualized on external examination. To transilluminate
the specimen, place the eye in front of a small in-tense light against a dark
background. One method is to use a substage microscope lamp in a dark room.
Rotate the globe over the light source, and look for areas of increased or
de-creased transmission of light. Increased trans-mission of light may be seen
in defects of the iris as occur in pigmentary dispersion syn-drome and
following peripheral iridectomy or cataract surgery. Decreased transmission of
light may be due to intraocular hemorrhage or intraocular tumors. Mark these
transilluminationdefects on the corresponding sclera with a pencil. The
location of these transillumination defects will determine how the eye should
be sectioned.
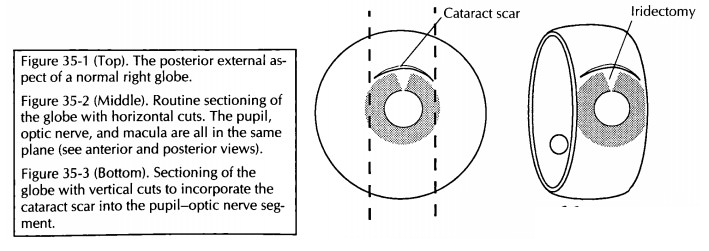
Sectioning
The
planes of section through the globe depend on the presence and location of a
lesion. Begin by cutting off the distal 3-mm portion of the optic nerve, and
submit this portion on end for histologic examination. If no focal lesions are
ap-parent after external examination, transillumina-tion, and review of the
clinical and surgical findings, open the eye in a horizontal plane par-allel to
the center of the optic nerve and macula. The ultimate aim is to provide a
median section along the pupil and optic nerve axis—the pupil– optic nerve
(P-O) section—which includes the pupil, optic nerve head, and macula in the
same section. Approaching the eye from its posterior aspect, place a razor
blade 5 mm superior to the center of the optic nerve. Do not aim the blade
toward the center of the pupil, as the lens is a hard structure that is easily
dislodged if you try to cut through it. Instead, avoid the lens by aiming 0.5
mm central to the limbus. Once the razor blade is engaged, turn the eye, and
view it from the side to help maintain the proper plane of sectioning. Using a
sawing motion, continue the section all the way through the limbus (Fig. 35-2).
Before
sectioning further, stop to examine the intraocular components. This
examination should be performed with a dissecting micro-scope. Systematically
evaluate the lens, iris, cili-ary body, vitreous, choroid, retina, and optic
nerve head. Note the size and location of any lesions. If an intraocular tumor
is present, docu-ment its location using the ora serrata, optic disk, and
macula as points of reference. Record its size in all dimensions. Try to
determine which ocular structures are involved. Specifically note the
rela-tionship of the tumor to the optic nerve.
Once the
intraocular examination is complete, finish sectioning the eye. Place the cut
surface of the globe on a flat surface, and then cut the eye in a plane
parallel to the initial section. Again, the razor blade should enter the
posterior aspect of the eye 5 mm from the center of the optic nerve, and it
should exit the anterior surface of the eye through the periphery of the cornea
(i.e., 0.5 mm central to the limbus). The completion of this second cut will
result in two caps (or cal-lottes) and the P-O segment.
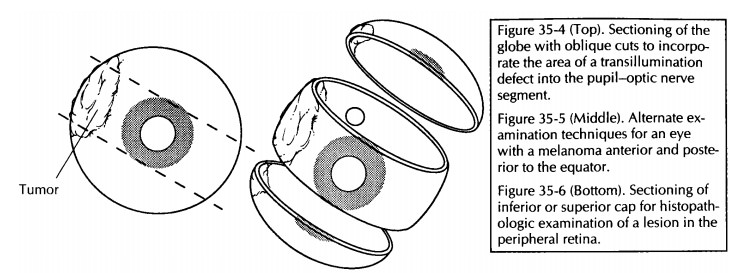
For eyes with focal lesions, the eye can be sec-tioned vertically or obliquely to include the lesion in the P-O plane. A few common examples are worthy of specific description. If evidence of prior cataract surgery is present, cut the globe ver-tically in a plane perpendicular to the wound (Fig. 35-3). If a corneal laceration is present, cut the globe perpendicular to the long axis of the lesion. If a transillumination defect (e.g., mela-noma) is present, cut the globe so that the center of the tumor is in the plane of the P-O segment (Fig. 35-4).
![]()
An
alternative technique for sectioning an eye with melanoma has been designed to
permit direct visualization of the tumor from the per-spective of the clinician
and thus enhance clinico-pathologic correlations.17 For
melanomas located posterior to the equator, the anterior segment (cornea, iris,
lens, and pars plicata) is removed en bloc by a coronal cut through the pars
plana just anterior to the ora serrata. For melanomas that extend anterior to
the equator, a cap is removed by a cut along the P-O plane. Either of these
cuts permits the examiner to look into the globe and directly visualize the
apex of the tumor (Fig. 35-5).
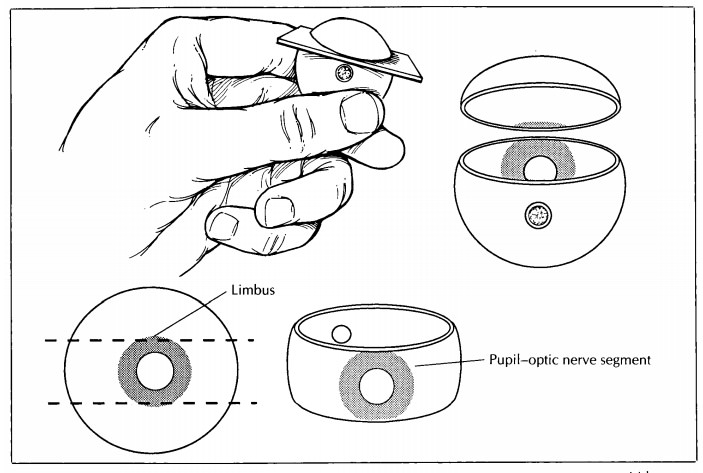
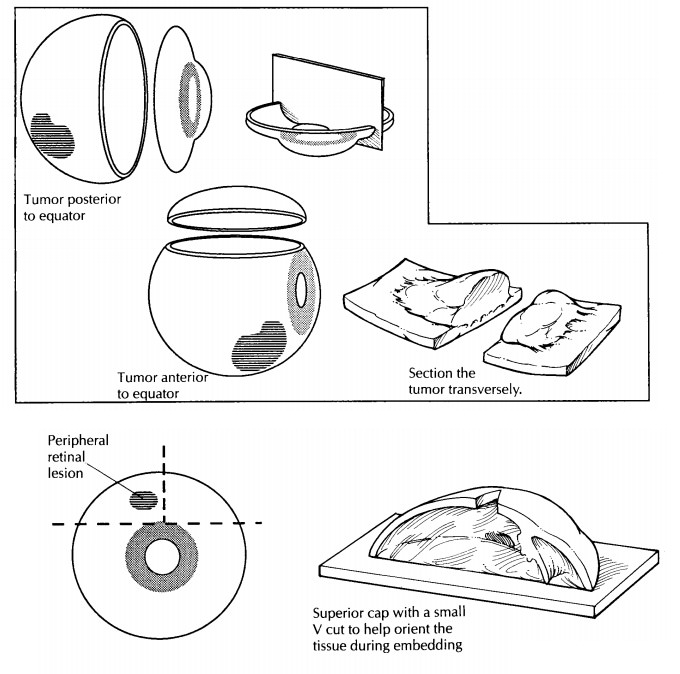
Tissue Sampling
In most
cases, only the P-O segment is submitted for histologic evaluation. Important
exceptions are when a cap contains the macula (as when the P-O segment is taken
in the vertical plane) or a lesion. If a lesion is present in the cap, then the
cap may be further sectioned into superonasal, superotemporal, inferonasal,
and/or inferotemp-oral segments, as necessary, and submitted for processing. A
mark on the tissue section and a note to the histotechnologist may help guide
proper orientation of the tissue during embed-ding. For example, a small V may
be cut into the segment opposite the margin of interest with instructions
provided to ‘‘embed V up’’ (Fig. 35-6). The pathologist may wish to supervise
the embedding of the tissue to ensure proper orienta-tion. Store any remaining
tissue in formalin, taking care to designate the tissue properly.
Cataract Specimens
The handling
of cataract specimens varies from institution to institution. In general,
cataractsare difficult to evaluate by light microscopy. Hence, the
histopathologic examination of cata-ract specimens is performed only for
special indications (such as with congenital cataracts and pseudoexfoliation
syndrome). If the sub-mission of cataract specimens is required, docu-ment the
color, diameter, and thickness of the lens nucleus.
Intracapsular
cataract extraction (ICCE) is a surgical technique that allows for the best
evalu-ation of the lens, as the lens is removed in its entirety (capsule,
cortex, and nucleus). ICCE was the procedure of choice for cataract extrac-tion
for many years; however, this technique is used today only in rare
circumstances (such as in conditions with zonular weakness).
Phacoemulsification
is a type of small-incision extracapsular cataract extraction (ECCE) that was
initially introduced in 1967. Recently, this technique has gained popularity
over the more traditional technique of ECCE. With phacoemul-sification, there
is a smaller incision, less induced astigmatism, and perhaps a faster recovery
of vision. The lens nucleus is emulsified ultrasoni-cally; therefore, no
cataract specimen is submitted.
Eyelid Excisions
Basal
cell carcinoma accounts for 85% to 95% of all malignant epithelial tumors of
the eyelids. Most excised specimens with basal cell carcinoma are elliptical.
If the long axis of the specimen is less than 10 mm, the specimen should be
bisected perpendicular to its long axis through the center of the tumor. This
technique allows for the evalu-ation of three surgical margins (two skin
margins and the deep margin). If the ellipse is larger than 10 mm, the specimen
can be cut in a cruciate configuration, resulting in a 3- to 4-mm central
portion and two end portions. The two end por-tions are bisected in a plane
perpendicular to the central portion. Separately label and submit the central
portion and end sections. With this tech-nique, all five surgical margins
(e.g., deep, lateral, medial, superior, and inferior) are evaluated.
Special Techniques
Given
the easy accessibility of the eye, small tis-sue samples for diagnostic
purposes are readilyobtained using a variety of methods. Although these
specimens do not require complex dissec-tions, they do require careful handling
to pre-serve cellular detail. Scrapings of the cornea and conjunctiva can be
processed as wet-fixed smears. This technique is useful in cases of
conjunctival intraepithelial neoplasia. Rapid fixation of the slides in 95%
ethyl alcohol is essential in this technique. Do not allow the specimen to air
dry. After fixation, the slides may be stained with a modified Papanicolaou
technique. Air-dried smears are often useful to look for infectious agents in
cases of suspected exogenous or endog-enous endophthalmitis. Drops of the
specimen are placed on the center of three or more slides and allowed to air
dry. The slides can then be fixed in 100% methanol for 5 minutes.
Micro-organisms can then be detected using Gram, Giemsa, periodic acid-Schiff
(PAS), and Papani-colaou stains.
The
Millipore filter preparation technique can be used to examine ocular fluid
specimens in cases of vitreous hemorrhage, proliferative vitreoretinopathy, and
suspected intraocular tumors. This procedure provides for excellent cytologic
preservation. The specimen may be received in a plastic syringe or a vitrectomy
cas-sette and must be fresh and unfixed before filtra-tion. After filtration,
the cells are fixed with 95% ethyl alcohol. Do not allow the filter to air dry
at any time during the procedure. If a specimen is very cellular, divide it
among several filters. During filtration, direct the washings along the sides
of the funnel to avoid disturbing the cells on the filter. Fix the filters for
a minimum of 15 minutes in a Petri dish with 95% ethyl alcohol. A modified
Papanicolaou technique, Gomori’s stain for iron, and the PAS stain are
routinely used to stain the Millipore filter. Absolute pro-pylalcohol rather
than absolute ethyl alcohol should be used during staining to avoid
dissol-ution of the filter. The stained Millipore filters are then mounted on
glass slides for micro-scopic examination.
The
celloidin bag technique is useful for the retrieval of tissue fragments and
cellular material suspended in a fluid. Place 10% neutral buffered formalin and
the specimen into a centrifuge-tube lined by a celloidin bag. Centrifuge for 10
minutes. Decant the supernatant, remove the celloidin bag, and tie the bag with
a string just above the pellet. Fix the specimen again in forma-lin for at
least 30 minutes. The specimen mayobtained using a variety of methods. Although
these specimens do not require complex dissec-tions, they do require careful
handling to pre-serve cellular detail. Scrapings of the cornea and conjunctiva
can be processed as wet-fixed smears. This technique is useful in cases of
conjunctival intraepithelial neoplasia. Rapid fixation of the slides in 95%
ethyl alcohol is essential in this technique. Do not allow the specimen to air
dry. After fixation, the slides may be stained with a modified Papanicolaou
technique. Air-dried smears are often useful to look for infectious agents in
cases of suspected exogenous or endog-enous endophthalmitis. Drops of the
specimen are placed on the center of three or more slides and allowed to air
dry. The slides can then be fixed in 100% methanol for 5 minutes.
Micro-organisms can then be detected using Gram, Giemsa, periodic acid-Schiff
(PAS), and Papani-colaou stains.
The
Millipore filter preparation technique can be used to examine ocular fluid
specimens in cases of vitreous hemorrhage, proliferative vitreoretinopathy, and
suspected intraocular tumors. This procedure provides for excellent cytologic
preservation. The specimen may be received in a plastic syringe or a vitrectomy
cas-sette and must be fresh and unfixed before filtra-tion. After filtration,
the cells are fixed with 95% ethyl alcohol. Do not allow the filter to air dry
at any time during the procedure. If a specimen is very cellular, divide it
among several filters. During filtration, direct the washings along the sides
of the funnel to avoid disturbing the cells on the filter. Fix the filters for
a minimum of 15 minutes in a Petri dish with 95% ethyl alcohol. A modified
Papanicolaou technique, Gomori’s stain for iron, and the PAS stain are
routinely used to stain the Millipore filter. Absolute pro-pylalcohol rather
than absolute ethyl alcohol should be used during staining to avoid
dissol-ution of the filter. The stained Millipore filters are then mounted on
glass slides for micro-scopic examination.
The
celloidin bag technique is useful for the retrieval of tissue fragments and
cellular material suspended in a fluid. Place 10% neutral buffered formalin and
the specimen into a centrifuge-tube lined by a celloidin bag. Centrifuge for 10
minutes. Decant the supernatant, remove the celloidin bag, and tie the bag with
a string just above the pellet. Fix the specimen again in forma-lin for at
least 30 minutes. The specimen may now be submitted for routine paraffin
processing and sectioning.
Important Issues to Address in Your Surgical Pathology Report on the Eye
•
What procedure was performed?
•
Is the specimen a right or left eye? What is
the size of the eye? (What are the anteropos-terior, horizontal, and vertical
dimensions? What is the length of the optic nerve? What are the horizontal and
vertical dimensions of the cornea?)
•
What is the status of the anterior segment
(surgical incisions, corneal opacification, iris or lens abnormalities)?
•
Are any transillumination defects present? What
are the measurements of these defects, and where are they in relation to
external land-marks?![]()
• Note the
condition of the iris, ciliary body, and lens. Is an intraocular lens present?
If so, is it in the anterior or posterior chamber? If in the posterior chamber,
is it in the capsular bag or in the sulcus (between the ciliary body and the
root of the iris)?
• Is there
a posterior vitreous or retinal detach-ment? Is any hemorrhage present in the
vitre-ous or retina? Is the choroid thickened? Is the optic nerve head cupped
or swollen?
• Is an
intraocular tumor present? Describe its type, location, size, color, margins,
and consistency. Is any associated hemorrhage or necrosis present? What ocular
structures are involved? Does the tumor extend into the optic nerve? Is the
tumor present grossly at the cranial or surgical margin of the optic nerve?
Related Topics