Occurrence of Ores, Physical and Chemical Properties, Uses - Extractive Metallurgy of Copper | 10th Science : Chapter 8 : Periodic Classification of Elements
Chapter: 10th Science : Chapter 8 : Periodic Classification of Elements
Extractive Metallurgy of Copper
EXTRACTIVE METALLURGY OF
COPPER
Occurrence:
It was named as cuprum
by the Romans because they got it from the Island of Cyprus. Copper is found in
the native state as well as combined state.
Ores of copper Formula
Copper pyrites CuFeS2
Cuprite or ruby copper Cu2O
Copper glance Cu2S
The chief ore of copper
is copper pyrite. It yields nearly 76% of the world production of copper.
Extraction of copper from copper pyrites involves the following steps
i. Concentration of ore: The ore is crushed and the concentrated
by froth floatation process.
ii. Roasting: The concentrated ore is
roasted in excess of air. During the process of roasting, the moisture
and volatile impurities are removed. Sulphur, phosphorus, arsenic and antimony
are removed as oxides. Copper pyrite is partly converted into sulphides of
copper and iron.
2CuFeS2 + O2 → Cu2S
+ 2 FeS + SO2 ↑
iii. Smelting: The roasted ore is mixed with powdered
coke and sand and is heated in a blast furnace to obtain matte (Cu2S
+ FeS) and slag. The slag is removed as waste.
iv. Bessemerisation: The molten matte is transferred to
Bessemer converter in order to obtain blister copper. Ferrous sulphide from
matte is oxidized to ferrous oxide, which is removed as slag using silica.
2 FeS + 3 O2 → 2 FeO + 2
SO2 ↑
FeO + SiO2 → FeSiO3
(slag) (Iron
silicate)
2 Cu2S + 3O2
→ 2 Cu2O + 2 SO2 ↑
2 Cu2O + Cu2S
→ 6 Cu + SO2↑ (Blister
copper)
v. Refining: Blister copper contains
98% of pure copper and 2% of impurities and is purified by electrolytic
refining. This method is used to get metal of a high degree of purity. For
electrolytic refining of copper, we use:
Cathode: A thin plate of pure
copper metal.
Anode: A block of impure
copper metal.
Electrolyte: Copper sulphate
solution acidified with sulphuric acid.
When electric current is
passed through the electrolytic solution, pure copper gets deposited at the
cathode and the impurities settle at the bottom of the anode in the form of
sludge called anode mud.
Physical Properties of Copper
Copper is a reddish
brown metal, with high lustre, high density and high melting point (1356°C).
Chemical Properties of Copper
i. Action of Air and Moisture: Copper gets covered
with a green layer of basic copper carbonate in the presence of CO2
and moisture.
2 Cu + O2 +
CO2 + H2O → CuCO3.Cu(OH)2
ii. Action of Heat: On
heating at different temperatures in the presence of oxygen, copper forms two
types of oxides CuO, Cu2O.
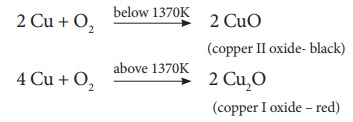
iii. Action of Acids:
a) With dilute HCl and
dilute H2SO4:
Dilute acids such as HCl
and H2SO4 have no action on these metals in the absence
of air. Copper dissolves in these acids in the presence of air.
2 Cu + 4 HCl + O2
(air) →2 CuCl2 + 2 H2O
b) With dil. HNO3:
Copper reacts with dil.
HNO3 with the liberation of Nitric Oxide gas.
3 Cu + 8 HNO3
→ Cu(NO3)2 + 2 NO ↑ + 2H2O
Cu + 2 H2SO4
→ CuSO4 + SO2 ↑ + 2 H2O
iv) Action of Chlorine:
Chlorine reacts with
copper, resulting in the formation of copper(II) chloride.
Cu + Cl2 ![]() CuCl2
CuCl2
v) Action of Alkalis:
Copper is not attacked
by alkalis.
Uses of Copper:
i.
It is extensively used in manufacturing electric cables and other
electric appliances.
ii.
It is used for making utensils, containers, calorimeters and
coins,
iii.
It is used in electroplating.
iv.
It is alloyed with gold and silver for making coins and jewels
Related Topics