Chapter: Modern Pharmacology with Clinical Applications: Principles of Toxicology
Exposure to Nontherapeutic Toxicants
EXPOSURE TO
NONTHERAPEUTIC TOXICANTS
Worldwide production of
chemicals has increased dra-matically in recent decades, resulting in increased
hu-man exposure. This applies not only to workers who manufacture the chemicals
and final products but also to those who use the products or are exposed
through contamination of surface and ground water and air.
Air Pollution
Industrial activity has
polluted the outdoor air with a number of chemicals known to be hazardous to
human health. These include a variety of gases, such as carbon monoxide, ozone,
and the oxides of sulfur and nitrogen. Unacceptable levels of air pollutants
can occur indoors as well. While some of these pollutants may be the same as
for the outdoor air, they also include biological agents (e.g., fungal spores,
viruses, bacteria, actino-mycetes), volatile organic compounds, carbon dioxide,
and formaldehyde.
Gases
Carbon monoxide arises from
the incomplete combus-tion of organic material. Of principal concern is its
gen-eration by the internal combustion engine and by home heating units,
particularly in poorly ventilated areas. Carbon monoxide emission by
automobiles in closed garages and by unvented space heaters results in
nu-merous deaths each year. Following inhalation, carbon monoxide binds to
hemoglobin, displacing oxygen and forming carboxyhemoglobin. This decreases the
oxy-gen-carrying capacity of the blood and impairs the blood cells’ ability to
release bound oxygen. The result-ing hypoxia is the principal mechanism of
carbon monoxide toxicity.
Nitrogen oxides, principally
nitrogen dioxide, and ozone are classified as oxidizing pollutants. The major
source of nitrogen dioxide is the internal combustion engine. Photolysis of
nitrogen dioxide by ultraviolet ra-diation liberates oxygen atoms, which can
then combine with molecular oxygen to form ozone. Both gases cause irritation
of the deep lung and can result in increased susceptibility to respiratory
infection, pulmonary edema, and impaired lung function.
Oxides of sulfur (principally sulfur dioxide) are gen-erated during the burning of fossil fuels, most notably coal, and are classified as reducing pollutants because of the types of reactions they undergo.
Particulate matter associated with most emissions promotes the conver-sion
of sulfur dioxide to the more toxic sulfuric acid and facilitates deposition in
the deep lungs. The acid can cause bronchospasm and lung damage, including
alve-olitis. Asthmatic episodes can be exacerbated by sulfur dioxide and
sulfuric acid.
Particulates
Industrial processes, such as
milling and mining, con-struction work, and the burning of wood or fossil fuel,
generate particulates that can be directly toxic or can serve as vectors for
the transfer of bound material, such as sulfuric acid, metals, and
hydrocarbons, into the lungs. Natural products such as pollen, anthrax spores,
and animal dander can elicit toxic reactions on inhala-tion or skin contact.
The inhalation of asbestos, silica, or coal dust can cause pneumoconiosis,
which may develop into serious lung disease. The size of the particle,
venti-latory rate, and depth of breathing will determine the extent of
pulmonary deposition.
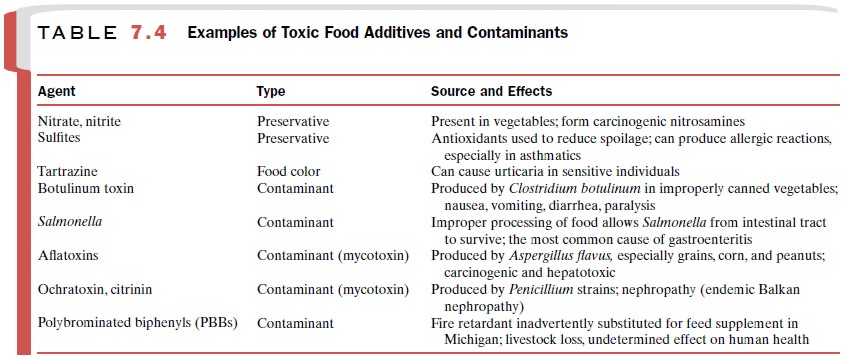
Food Additives and Contaminants
Thousands of substances are
added to foods to enhance their marketability (appearance, taste, texture,
etc.), storage properties, or nutritive value, any of which may cause toxicity
in susceptible individuals (Table 7.4). Microbial or fungal contamination of
food, either dur-ing processing or storage, can introduce potent toxins into food.
Metals
Characteristics of toxicity for a number of metals are presented in Table 7.5. While the exact tissue and mo-lecular site of the toxic action of each metal is different, toxicity generally results from interaction of the metal with specific functional groups on macromolecules in the cell. These groups include sulfhydryl, carboxyl, amino, phosphoryl, and phenolic moieties. Interactions of such groups with metals can lead to disruption of en-zyme activities and transport processes and eventually to loss of such cellular functions as energy production and ion regulation.
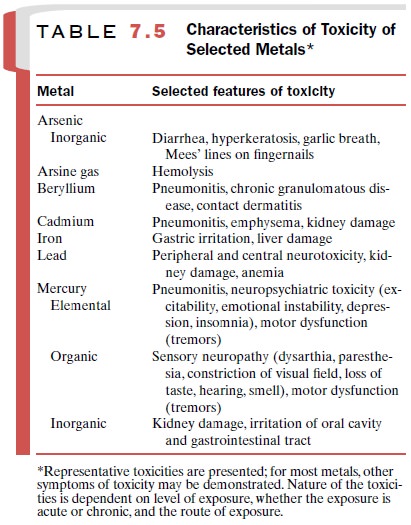
In general, toxicity is
related to the form of the metal (inorganic, organic, or elemental), the route
of exposure, and the route of excretion.
Solvents
Solvents are generally
classified as aliphatic or aro-matic, and either type may be halogenated, most
com-monly with chlorine. The toxicity of representative sol-vents is summarized
in Table 7.6. Occupational expo-sure to solvents occurs in cleaning, degreasing,
painting, and gluing. Exposure to solvents is generally through in-halation of
vapors, although direct skin contact also oc-curs. The concentration of solvent
in air is determined by the vapor pressure of the solvent, the ambient
tem-perature, and the effectiveness of ventilation systems. These factors and
the rate of pulmonary air exchange will affect the extent of exposure. Sniffing
glue fumes is one form of substance abuse.
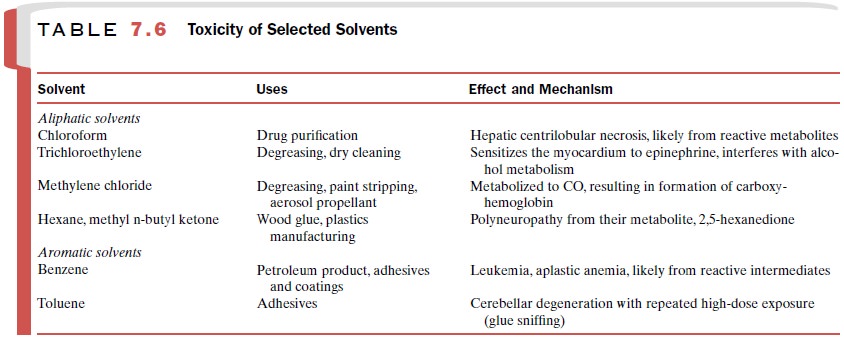
Solvents are generally
lipid-soluble, and therefore they are readily absorbed across the skin. Once
ab-sorbed, they tend to concentrate in the brain, and CNS dysfunction is common
at high exposures. Symptoms can range from confusion to unconsciousness.
Solvents often undergo bioactivation and may cause systemic toxicity as a result
of the formation of reactive interme-diates.
Pesticides
Pesticides are chemicals used
to eliminate unwanted or-ganisms. Common targets for pesticides include
insects, weeds (herbicides), fungi, and rodents. Poisoning from pesticides
often affects professional exterminators, agri-cultural workers, and consumers
(Table 7.7). More than half of the poisonings due to agricultural pesticides
af-fect children.
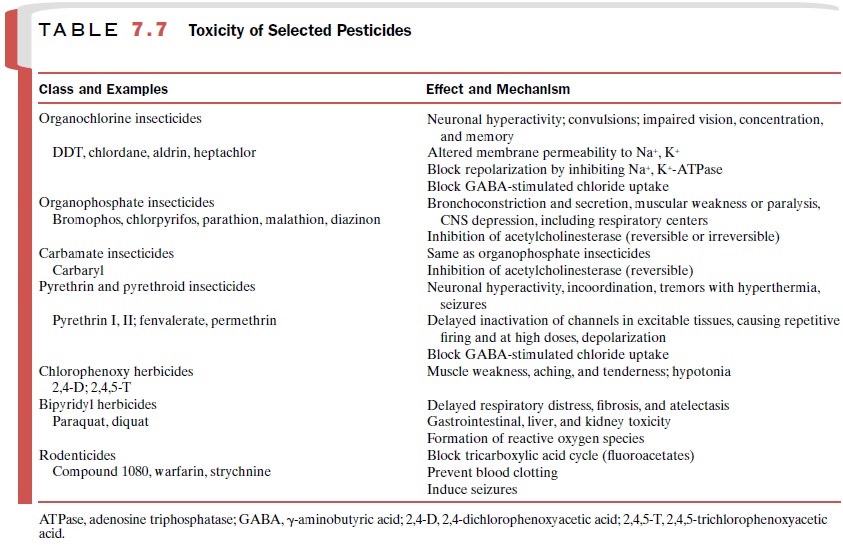
Insecticides
The prototypical organochlorine insecticide is DDT. It
was first used in World War II for vector control of malaria. The
organochlorine insecticides are very stable in the environment. This
persistence allows toxic con-centrations to build up in nontarget organisms.
Organophosphate insecticides (e.g., malathion, parathion, diazinon) undergo metabolic activation to yield an oxygenated
metabolite that will react with the active site of acetylcholinesterase (AChE),
resulting in irreversible enzyme inhibition. Symptoms of poisoning are due to
excessive stimulation of cholinergic recep-tors. In cases of lethal poisoning
in humans, death is from respiratory failure. Distal neuropathy of the lower
limbs also has been seen.
The carbamate insecticides also inhibit AChE. The mechanism of
inhibition is similar, but the reaction is re-versible.
Herbicides and Rodenticides
Herbicidal activity generally
consists of interference with plant-specific biochemical reactions. Thus,
mam-malian toxicity is generally low and not predictable from the mechanism of
herbicidal action. In contrast, rodenticide target selectivity is not based on
differences in biochemistry between humans and rodents but rather on
differences in physiology or behavior, especially feeding behavior. For
example, an emetic may be in-cluded in a rodenticide formulation to promote vomit-ing
in humans who accidentally consume the product; rodents do not have a vomit
reflex.
The chlorophenoxy herbicides, 2,4-dichlorophe-noxyacetic acid (2,4-D)
and 2,4,5-trichlorophenoxy-acetic acid (2,4,5-T), were used in defoliating
operations in Vietnam, and the adverse health effects of the con-taminant
2,3,7,8-tetrachlorodibenzodioxin (dioxin) con-tinue to be controversial.
The bipyridyl herbicides paraquat and diquat are broad-spectrum
herbicides. As little as 10 mL of paraquat concentrate is lethal in adults.
Paraquat dam-ages the lungs and may result in the appearance of a respiratory
distress syndrome appearing 1 or 2 weeks after poisoning. In contrast, diquat
causes minimal lung damage because it does not selectively accumulate in the
lung. Acute renal failure, liver toxicity, and gastroin-testinal damage are
sequelae to diquat poisoning.
Warfarin, a coumarin
anticoagulant, is incorporated into cornmeal for use as a rat poison. Repeated
expo-sure results in sufficient inhibition of prothrombin syn-thesis to cause
fatal internal hemorrhage.
Related Topics