Chapter: Modern Analytical Chemistry: Uality Assurance
Evaluating Quality Assurance Data: Prescriptive Approach
Evaluating Quality
Assurance Data
In the previous
section we described several internal methods
of quality assessment that provide quantitative estimates of the systematic and random errors
present in an analytical system. Now we turn our attention to how this numerical information is incorporated into the written directives of a complete
quality assurance program. Two approaches to developing quality
assurance programs have been described9: a
prescriptive approach, in which an exact method
of quality assessment is prescribed; and a performance-based approach, in which any form of quality assessment is ac- ceptable, provided
that an acceptable level of statistical control can be demonstrated.
Prescriptive Approach
With a prescriptive approach to quality
assessment, duplicate samples,
blanks, stan- dards, and
spike recoveries are measured following a specific protocol. The result for each analysis is then compared
with a single predetermined limit. If this limit is exceeded, an appropriate corrective action is taken.
Prescriptive approaches to qual-
ity assurance are common for programs and laboratories subject
to federal regula- tion. For example, the Food and Drug Administration (FDA) specifies quality
as- surance practices that must be followed by laboratories analyzing products
regulated by the FDA.
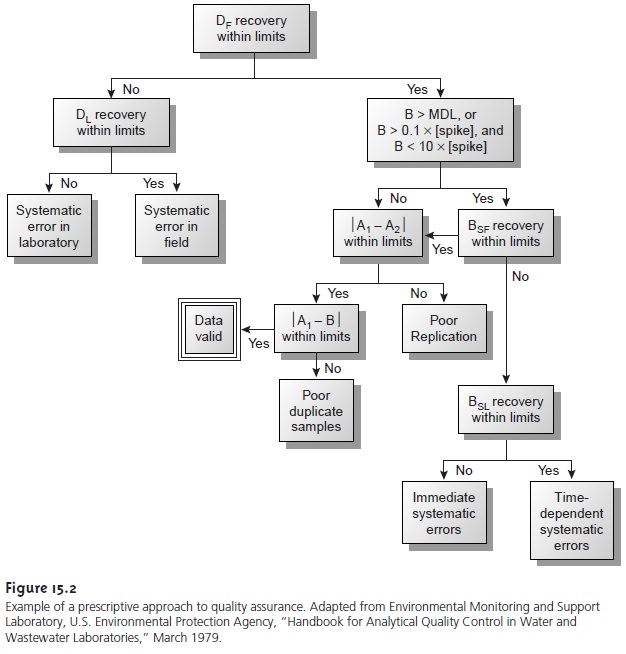
A good example
of a prescriptive approach to quality assessment is the protocol outlined in Figure 15.2,
published by the Environmental Protection Agency (EPA) for laboratories involved
in monitoring studies
of water and wastewater.10 Indepen- dent samples
A and B are collected simultaneously at the sample site.
Sample A is split
into two equal-volume samples, and labeled
A1 and A2. Sample B is also split into two equal-volume samples,
one of which, BSF, is spiked with a known
amount of analyte. A field blank,
DF, also is spiked with the same amount of analyte. All five
samples (A1, A2, B, BSF, and DF) are preserved if necessary and transported to the
laboratory for analysis.
The first sample
to be analyzed is the field blank.
If its spike recovery is unac-
ceptable, indicating that
a systematic error
is present, then
a laboratory method
blank, DL, is prepared and analyzed. If the spike
recovery for the method blank
is also unsatisfactory, then the systematic error originated in the laboratory. An ac- ceptable spike
recovery for the
method blank, however, indicates that the
systematic error occurred in the field or during transport to the laboratory. Systematic errors in the laboratory can be corrected, and the analysis
continued. Any systematic er- rors occurring in the field, however, cast uncertainty on the quality
of the samples, making it necessary
to collect new samples.
If the field blank is satisfactory, then sample B is analyzed. If the result for B is above the method’s detection limit, or if it is within the range of 0.1 to 10 times the amount of analyte spiked into BSF, then a spike recovery for BSF is determined.
An unacceptable spike recovery
for BSF indicates the presence of a systematic error in- volving the
sample. To determine the source of the systematic error, a laboratory spike, BSL, is prepared
using sample B and analyzed.
If the spike recovery for BSL is acceptable, then the systematic error requires a long time to have a noticeable effect on the spike
recovery. One possible explanation is that
the analyte has
not been properly preserved or has been held beyond
the acceptable holding
time. An unac- ceptable spike recovery for BSL suggests
an immediate systematic error, such as that
due to the influence of the sample’s
matrix. In either case, the systematic errors are
fatal and must be corrected before the sample
is reanalyzed.
If the spike
recovery for BSF is acceptable, or if the
result for sample
B is below the method’s detection limit or outside
the range of 0.1 to 10 times
the amount of analyte spiked in BSF, then the duplicate samples A1 and
A2 are analyzed. The results for A1 and A2 are discarded if the difference between their values is excessive. If the difference between
the results for A1 and A2 is within the accepted limits,
then the results for samples A1 and B are compared. Since samples collected
from the same sampling site at the same time should be identical in composition, the results are discarded if the difference between their values
is unsatisfactory, and
accepted if the difference is satisfactory.
This protocol requires
four to five evaluations of quality assessment data before the result
for a single sample can
be accepted; a process that
must be repeated for each analyte and
for each sample.
Other prescriptive protocols are equally demand- ing. For example, Figure 3.7 shows a portion
of the quality assurance
protocol used for the graphite
furnace atomic absorption analysis of trace metals in aqueous solutions. This protocol
involves the analysis
of an initial calibration verifi- cation standard and an initial calibration blank, followed by the analysis
of samples in groups of ten. Each group of samples
is preceded and followed by continuing cal- ibration verification (CCV) and continuing calibration blank (CCB) quality
assess- ment samples. Results
for each group
of ten samples
can be accepted only if both sets of CCV and CCB quality
assessment samples are acceptable.
The advantage to a prescriptive approach to quality
assurance is that a single
con- sistent set of guidelines is used by all laboratories to control the
quality of analytical results. A significant disadvantage, however, is that the ability
of a laboratory to pro- duce quality results is not taken
into account when
determining the frequency of col- lecting and analyzing quality
assessment data. Laboratories with a record of producing high-quality results
are forced to spend more time and money on quality assessment than is perhaps necessary. At the same
time, the frequency of quality assessment may be insufficient for laboratories with
a history of producing results
of poor quality.
Related Topics