Volumetric Analysis | Chemistry Practical Laboratory Experiment - Estimation of Ferrous Sulphate | 12th Chemistry : Practicals
Chapter: 12th Chemistry : Practicals
Estimation of Ferrous Sulphate
VOLUMETRIC ANALYSIS
Estimation of
Ferrous Sulphate (Fe2+)
Aim :
To
estimate the amount of ferrous sulphate dissolved in 750 ml of the given
unknown solution volumetrically. For this you are given with a standard
solution of ferrous ammonium sulphate (FAS) of normality 0.1102 N and potassium
permanganate solution as link solution.
Principle:
During
these titrations, Fe2+ ions (from ferrous salts) are oxidised to MnO4-
ions and MnO4- ion (from Mn2+) is reduced to
Mn2+ ion.
Oxidation : 5
Fe2+ →
5 Fe3+ + 5e−
Reduction : 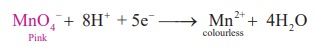
Overall reaction:
5Fe2+
+ MnO4- + 8H → 5Fe3+ + Mn2+ + 4H2O
Short procedure :
Procedure :
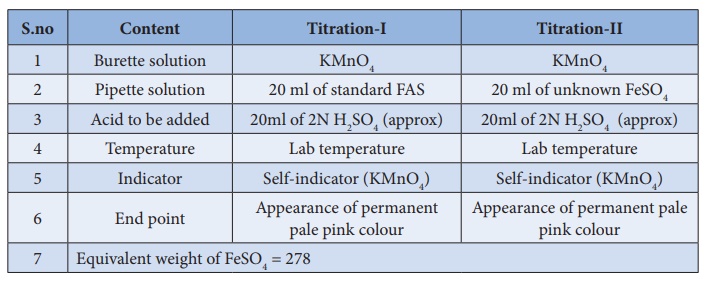
Titration–I
(Link
KMnO4)Vs (Standard FAS)
Burette
is washed with water, rinsed with KMnO4 solution and filled with
same KMnO4 solution up to the zero mark. Exactly 20 ml of standard
FAS solution is pipetted out into the clean, washed conical flask. To this FAS
solution, approximately 20ml of 2N sulphuric acid is added. This mixture is
titrated against KMnO4 Link solution from the burette. KMnO 4
is added drop wise till the appearance of permanent pale pink colour. Burette
reading is noted, and the same procedure is repeated to get concordant values.
Titration –I
(Link
KMnO4 )Vs (Standard FAS)
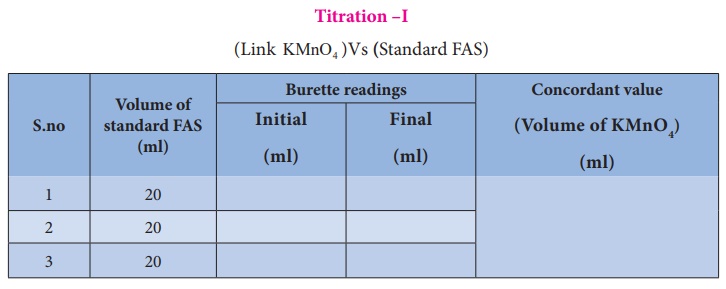
Calculation :
Volume of
KMnO4 (link) solution (V1) = ----------ml
Normality
KMnO4 (link) solution (N1) =-----------N
Volume of
standard FAS solution (V2) = 20 ml
Normality
of standard FAS solution (N2) = 0.1102 N
According
to normality equation: V1× N1 = V2 × N2
N1
= V2 × N2 / V1
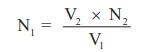
Normality
of KMnO4 (link) solution (N1) = ----------------- X N
Titration–II
(Unknown FeSO4 ) Vs
(Link KMnO4)
Burette
is washed with water, rinsed with KMnO4 solution and filled with same
KMnO4 solution up to the zero mark. Exactly 20 ml of unknown FeSO4
solution is pipetted out into the clean, washed conical flask. To this FeSO4
solution approximately 20ml of 2N sulphuric acid is added. This mixture is
titrated against KMnO4 Link solution from the burette. KMnO4
is added drop wise till the appearance of permanent pale pink colour. Burette
reading is noted and the same procedure is repeated to get concordant values.
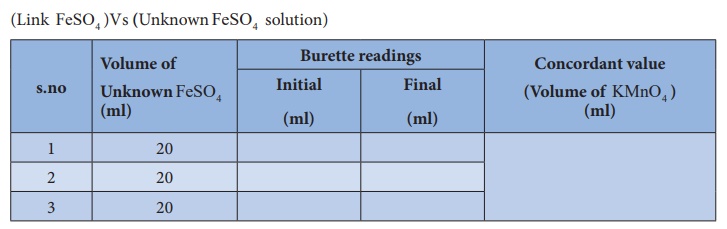
Titration –II
(Link
FeSO4 )Vs (Unknown FeSO4
solution)
Calculation :
Volume of
Unknown FeSO4 solution V1
= 20 ml
Normality
of Unknown FeSO4 solution N1 = ? N
Volume of
KMnO4 (link) solution V2 = ml
Normality
KMnO4 (link) solution N2
= X N
According
to normality equation: V1× N1 = V2 × N2
N1 = V2 × N2 / V1
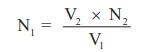
N1=
--------Y--------- N
The
normality of unknown FeSO4
solution = ________________ N
Weight calculation:
The
amount of FeSO4 dissolved in 1 lit of the solution = (Normality) x
(equivalent weight)
The
amount of FeSO4 dissolved in 750 ml of the solution = Normality x equivalentweight x 750 / 1000
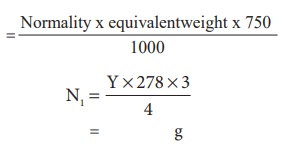
Report :
The
amount of FeSO4 dissolved in 750 ml of the solution = ---g
Related Topics