Chapter: Biochemistry: The Behavior of Proteins: Enzymes
Enzyme Kinetic Equations
Enzyme Kinetic Equations
The rate
of a chemical reaction is usually expressed in terms of a change in the
concentration of a reactant or of a product in a given time interval. Any
convenient experimental method can be used to monitor changes in concentration.
In a reaction of the form A + B - > P, where A and B are reactants and P is the
product, the rate of the reaction can be expressed either in terms of the rate
of disappearance of one of the reactants or in terms of the rate of appearance
of the product. The rate of disappearance of A is - Ōłå[A]/
Ōłå t, where symbolizes
change, [A] is the concentration of A in moles per liter, and t is time. Likewise, the rate of
disappearance of B is - Ōłå [B]/ Ōłåt, and the rate of appearance of P is Ōłå [P]/ Ōłåt. The rate of the reaction can be expressed in
terms of any of these changes because the rates of appearance of product and
disappearance of reactant are related by the stoichiometric equation for the
reaction
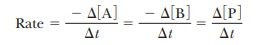
The
negative signs for the changes in concentration of A and B indicate that A and
B are being used up in the reaction, while P is being produced.
It has
been established that the rate of a reaction at a given time is pro-portional
to the product of the concentrations of the reactants raised to the appropriate
powers,

where k is a proportionality constant called
the rate constant. The exponents f and
g must be determined experimentally. They are not necessarily equal to thecoef├×cients of the balanced equation,
but frequently they are. The square brackets, as usual, denote molar
concentration. When the exponents in the rate equation have been determined
experimentally, a mechanism for the reactionÑa description of the detailed
steps along the path between reactants and productsÑcan be proposed.
The
exponents in the rate equation are usually small whole numbers, such as 1 or 2.
(There are also some cases in which the exponent 0 occurs.) The values of the
exponents are related to the number of molecules involved in the detailed steps
that constitute the mechanism. The overall
order of a reaction is the sum of all the exponents. If, for example, the
rate of a reaction A 3 P is given by the rate equation

where k is the rate constant and the exponent
for the concentration of A is 1, then the reaction is first order with respect to A and ├×rst order overall. The rate of
radioactive decay of the widely used tracer isotope phosphorus 32 (32P;
atomic weight = 32) depends only on the concentration of 32P
present. Here we have an example of a ├×rst-order reaction. Only the 32P atoms
are involved in the mechanism of the radioactive decay, which, as an equation,
takes the form

where k is the rate constant, the exponent for
the concentration of A is 1, and the exponent for the concentration of B is 1,
then the reaction is said to be ├×rst order with respect to A, ├×rst order with
respect to B, and second order
overall. In the reaction of glycogenn (a
polymer of glucose with n glucose
residues) with inorganic phosphate, Pi, to form
glucose 1-phosphate + glycogenn-1, the
rate of reaction depends on the concentrations of both reactants.
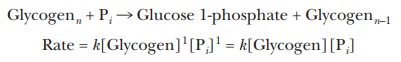
where k is the rate constant. Both the
glycogen and the phosphate take part in the reaction mechanism. The reaction of
glycogen with phosphate is ├×rst order with respect to glycogen, ├×rst order with
respect to phosphate, and second order overall.
Many
common reactions are first or second order. After the order of the reaction is
determined experimentally, proposals can be made about the mechanism of a
reaction.
Is the rate of a reaction always based on the concentration of reactants?
Exponents
in a rate equation may be equal to zero, with the rate for a reaction A - >
B given by the equation

Such a
reaction is called zero order, and
its rate, which is constant, depends not on concentrations of reactants but on
other factors, such as the presence of catalysts. Enzyme-catalyzed reactions
can exhibit zero-order kinetics when the concentrations of reactants are so
high that the enzyme is completely saturated with reactant molecules. This
point will be discussed in more detail later but, for the moment, we can
consider the situation analogous to a traf├×c bottleneck in which six lanes of
cars are trying to cross a two-lane bridge. The rate at which the cars cross is
not affected by the number of waiting cars, only by the number of lanes
available on the bridge.
Summary
The rate of a chemical reaction is measured by
the rate of appearance of the products or the rate of disappearance of the
substrates.
The rate of a reaction is mathematically equal
to a rate constant, k, multi-plied by
the concentration of substrate(s) raised to an exponent.
The order of a reaction is described by the
exponent in the rate equation. Common reaction orders are zero order, first
order, and second order.
The rate
constant, k, and the exponents must
be measured experimen-tally for each reaction.
Related Topics