Chapter: Biochemistry: Glycolysis
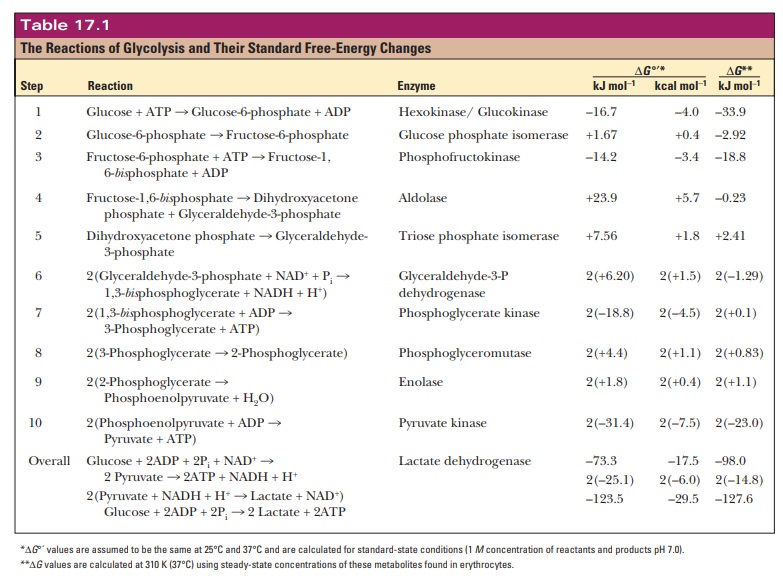
Energy Production in Glycolysis
Energy Production in Glycolysis
What is the energy yield from glycolysis?
Now that
we have seen the reactions of the glycolytic pathway, we can do some
bookkeeping and determine the standard free-energy change for the entire
pathway by using the data from Table 17.1.
The
overall process of glycolysis is exergonic. We can calculate ∆G°' for the entire reaction by adding
up the ∆G°' values from each of the
steps. Remember that all of the reactions from triose phosphate isomerase to
pyruvate kinase are doubled. This gives a final figure from glucose to two
pyruvates of –74.0 kJ mol1 or –17.5 kcal mol–1. The
energy released in the exergonic phases of the process drives the endergonic reactions.
The net reaction of glycolysis explic-itly includes an important endergonic
process, that of phosphorylation of two molecules of ADP.
2ADP +
2Pi - > 2ATP
∆G°' reaction = 61.0 kJ mol–1= 14.6
kcal mol–1glucose consumed
Without
the production of ATP, the reaction of one molecule of glucose to produce two
molecules of pyruvate would be even more exergonic. Thus, subtracting out the synthesis of ATP:
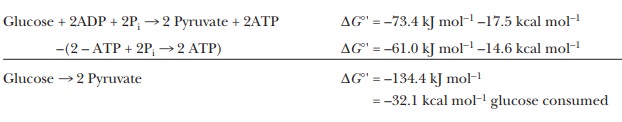
(The
corresponding figure for the conversion of one mole of glucose to two moles of
lactate is –184.6 kJ mol–1 = –44.1 kcal mol–1.) Without
production of ATP, the energy released by the conversion of glucose to pyruvate
would be lost to the organism and dissipated as heat. The energy required to
produce the two molecules of ATP for each molecule of glucose can be recovered
by the organism when the ATP is hydrolyzed in some metabolic process. We
discussed this point briefly, when we compared the thermodynamic efficiency of
anaerobic and aerobic metabolism. The percentage of the energy released by the
breakdown of glucose to lactate that is “captured” by the organism when ADP is
phosphorylated to ATP is the efficiency of energy use in glycolysis; it is
(61.0/184.6) X100, or about 33%. It comes from calculating the energy used to
phosphorylate two moles of ATP as a percentage of the energy released by the
conversion of one mole of glucose to two moles of lactate. The net release of
energy in glycolysis, 123.6 kJ (29.5 kcal) for each mole of glucose converted
to lactate, is dissipated as heat by the organism. Without the production of ATP
to serve as a source of energy for other metabolic processes, the energy
released by glycolysis would serve no purpose for the organism, except to help
maintain body temperature in warm-blooded animals. A soft drink with ice can
help keep you warm even on the coldest day of winter (if it is not a diet
drink) because of its high sugar content.
The
free-energy changes we have listed are the standard val-ues, assuming the
standard conditions, such as 1 M
concentrations of all solutes except hydrogen ion. Concentrations under
physiological conditions can dif-fer markedly from standard values.
Fortunately, there are well-known methods
for calculating the difference in the free-energy change. Also, large changes
in concentrations frequently lead to relatively small differences in the
free-energy change, about a few kilojoules per mole. Some of the free-energy
changes may be different under physiological conditions from the val-ues listed
here for standard conditions, but the underlying principles and the conclusions
drawn from them remain the same.
Summary
Glycolysis is an exergonic process, releasing 73.4 kJ for every
mole of glu-cose converted to two moles of pyruvate, accompanied by
phosphoryla-tion of two moles of ADP to ATP.
Without the production of ATP, glycolysis would be even more
strongly exergonic
Related Topics