Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Elevation of boiling point of dilute solutions and Cottrell's Method
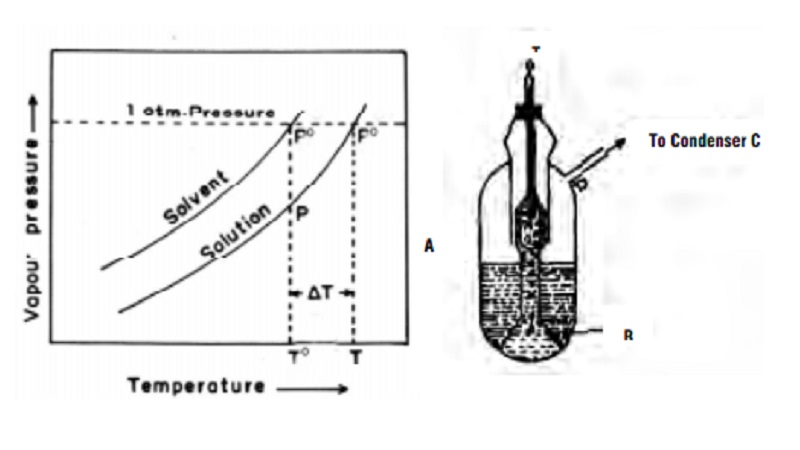
Elevation of boiling point of dilute solutions
The boiling point of a pure liquid is the temperature at which its
vapour pressure becomes equal to the atmospheric pressure. Since the vapour
pressure of a solution is always lower than that of the pure solvent, it
follows that the boiling point of a solution will always be higher than of the
pure solvent.
In the Fig., the upper curve represents the vapour pressure -
temperature dependance of the pure solvent. The lower curve represents the
vapour pressure - temperature dependance of a dilute solution with known
concentration. It is evident that the vapour pressure of the solution is lower
than that of the pure solvent at every temperature. The temperature To
gives the boiling point of the pure solvent and T the boiling point of the pure
solution. This is because at these temperatures (To, T) the vapour
pressures of pure solvent and solution becomes equal to the atmospheric
pressure.
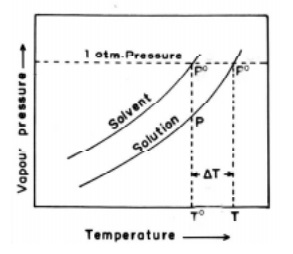
The elevation of boiling point = ∆Tb = T - T0
Elevation of boiling point is found directly proportional to the
molality of the solution (or) inturn the number of molecules of solute. Also it
is independent of the nature of the solute for a non-volatile solute. Hence,
boiling point elevation is a colligative property.
Thus it may be written as
∆Tb prop to m
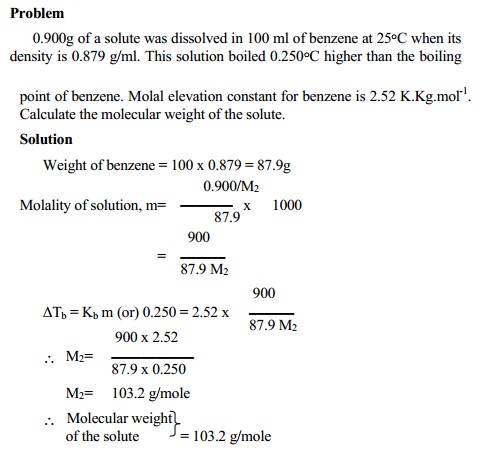
Determination of molecular weight from boiling
point elevation
By measuring the boiling point elevation of a solution of a known
concentration, it is possible to calculate molecular weight of a non-volatile
non-electrolyte solute.
∆Tb prop to m
∆Tb = Kb m
The proportionality constant Kb is characteristic of the solvent and it
is called the molal boiling point
elevation constant or ebullioscopic
constant. It is defined as the
elevation of boiling point of one molal solution.
When n2 moles of the solute is dissolved in W1 kg
of the solvent, the molality is given by n2/W1.
∆Tb = Kb W2 / M2W1
Since W2, is the weight of the
solute, we can calculate the molecular weight of the solution using the
following expression.
M2 = Kb . W2
/ ∆TbW1
Molal Elevation (Ebullioscopic) constants (One
mole of solute per 1000 grams of solvent)
Solvent B.
Pt K Kb (K.kg.mole-1)
Water 373.00 0.52
Benzene 353.10 2.57
Methanol 337.51 0.81
Ethanol 351.33 1.20
Carbon
tetra chloride 349.72 5.01
Chloroform 334.20 3.88
Acetic
acid 391.50 3.07
Acetone 329.15 1.72
Carbon
disulphide 319.25 2.41
Phenol 455.10 3.56
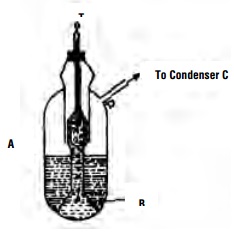
Determination of elevation of boiling point by
Cottrell's Method
The apparatus (Fig.) consists of a boiling tube (a) which is graduated
and contains weighed amount of the liquid under examination. An inverted funnel
tube (b) placed in the boiling tube collects the bubbles rising from a few
fragments of a porous pot placed inside the liquid. When the liquid starts
boiling, it pumps a stream of a liquid and vapour over the bulb of the Beckmann
thermometer (f) held a little above the liquid surface. In this way, the bulb
is covered with a thin layer of boiling liquid which is in equilibrium with the
vapour. This ensures that the temperature reading is exactly that of the
boiling liquid and that superheating is minimum. After determining the boiling
point of the pure solvent, a weighed amount of the solute is added and procedure
is repeated for another reading. The vapours of the boiling liquid is cooled in
a condenser (C) which has circulation of water through (d) and (e). The cooled
liquid drops into the liquid in (a).
Related Topics