Chapter: 11th Biochemistry : Chapter 10 : Biochemical Techniques
Electrophoresis: Principle and Types
Electrophoresis
In 1948,
a Swedish Physical Biochemist, Arne Tiselius was awarded the Nobel prize in
chemistry for the discovery of proteins in blood serum and for studying the
properties of proteins through electrophoresis. Till now, electrophoresis
continues to be an important technique to identify and characterize biological
macromolecules. Amino acids, peptides, proteins and nucleic acids possess
ionisable groups and can be made to exist in solution as cations or anions.
When a mixture of these components is subjected to an electric field, they
migrate differently and can be separated.
Principle
Electrophoresis
is defined as the migration of charged particles, under the influence of an
electric field at a definite pH. In a mixture of proteins, each protein with
its electrical charge will move differently in an electric field. This
electrophoretic mobility depends on the pH of the medium, strength of the
field, net charge of the molecule and size/shape of the molecule.
Electrophoresis is used for the analysis of large molecules (proteins and
nucleic acids) and simpler charged molecules (peptide, simpler ions).
Types of electrophoresis
The
following are different types or electrophoresis.
1. Paper electrophoresis
2. Cellulose acetate electrophoresis
3. Capillary electrophoresis
4. Gel electrophoresis
Agarose
gel electrophoresis, Polyacrylamide Gel Electrophoresis (SDS PAGE, Native PAGE
and two- dimensional electrophoresis).
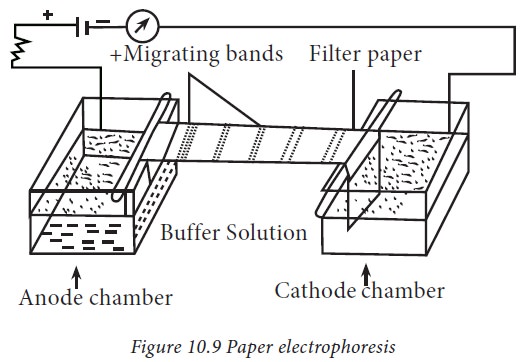
1. Paper electrophoresis
Paper
electrophoresis is an inexpensive method and requires only micro-quantities of
protein. The apparatus consists of two troughs to accommodate a buffer through
which an electric current is applied (Fig.10.9). Paper is a popular support
medium as it is easy to handle, less expensive and is readily available. Paper
contains 98% of cellulose. Paper electrophoresis has potential limitations. The
greatest problem is the thickness and large pore size of the paper. The
separation of proteins by paper electrophoresis takes longer time which limits
its use.
2. Gel Electrophoresis
i. Polyacrylamide Gel Electrophoresis
Polyacrylamide
gel is prepared from acrylamide and bis-acrylamide in a suitable buffer.
Polymerization of Acrylamide and bisacrylamide is achieved by a free radical
reaction promoted by N,N,N’,N’tetramethylethylenediamine (TEMED). This free
radical process is initiated by Ammonium per sulfate (APS) used in gel.
Acrylamide and bisacrylamide monomers are weak neurotoxin whereas, the
polymerised polyacrylamide is non-toxic. While handling acrylamide solutions,
care should be exercised and spectacles, gloves and mask should be worn.
ii. Sodium dodecyl Sulphate (SDS) polyacrylamide gel electrophoresis
Sodium
dodecyl Sulphate Polyacrylamide Gel Electrophoresis(SDS-PAGE) is an
electrophoretic technique very commonly used in Biochemistry, Molecular biology
and forensic science. This technique was first described by Laemmli in the year
1970 and till now dominates in scientific research.
Electrophoresis apparatus: The
electrophoretic apparatus consists of a reservoir tank to fill running buffer,
transparent insulating cover, gel plates, spacers and gel comb to form wells.
Platinum electrodes provide even current with the help of a regulated power
pack. The gel is packed in-between two glass plates with the help of spacers.
Clear wells are obtained using comb. Samples are layered in the little slots
cut in the top of the gel slab using gel comb. Buffer is cautiously layered
over the samples, and a voltage is applied to the gel using power pack for a
period of usually 1-3 h. The proteins migrate in the gel depending upon their
electrophoretic mobility, which is dependent on the size.

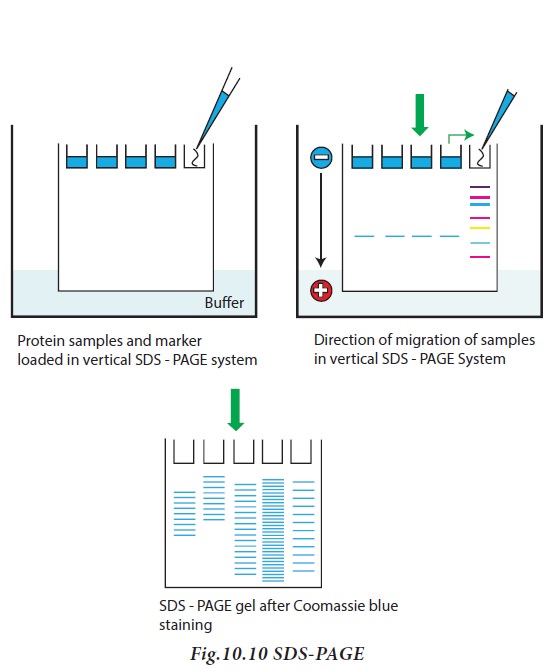
Protein samples to be run on SDS-PAGE
are added to sample solubilizing buffer containing beta mercaptoethanol
(disrupt disulphide bridges), SDS, glycerol (to make the solution denser and
enable proteins to sink in the gel) and bromophenol blue (tracking dye).
SDS -PAGE contain resolving gel, used
for separation of proteins and stacking gels for concentrating the proteins
prior to entry into resolving gel. Sodium dodecyl sulphate (SDS) is an anionic
detergent, which binds to proteins, and provides a constant negative charge per
unit mass. Protein-SDS complexes will therefore move towards the anode during
electrophoresis and their mobilities are inversely proportional to the log of their
molecular weights. Since the SDS impart proteins have the same charge per unit
length, all proteins travel with the same mobility. However, as the mixture of
proteins pass through the resolving gel, the proteins separate, owing to the
molecular sieving properties of the gel. The smaller proteins move fast as they
can pass through the pores of the gel. But larger proteins move slowly since
they are retarded by frictional resistance due to sieving effect of the gels.
When the dye reaches the bottom of the gel, the current is turned off. After
electrophoresis, the gel is carefully removed from the glass plate, immersed in
buffer and stained with appropriate stain solution (Fig.10.10)
Protein staining: Proteins can be
detected using Coomassie Brilliant Blue G250 (CBB) solution. CBB dye stains protein with a detection limit of
40μg. For proteins of less quantity, another sensitive detection known as
silver staining (1-5ng detection limit), can be performed.
Applications : SDS–PAGE is used to
determine the molecular weight of proteins.
To achieve this, a standard mixture of proteins of various molecular weight
(molecular weight ladder) was added for direct comparison of migration
distance. The molecular weights around 15-200kDa can be analyzed in this
manner.
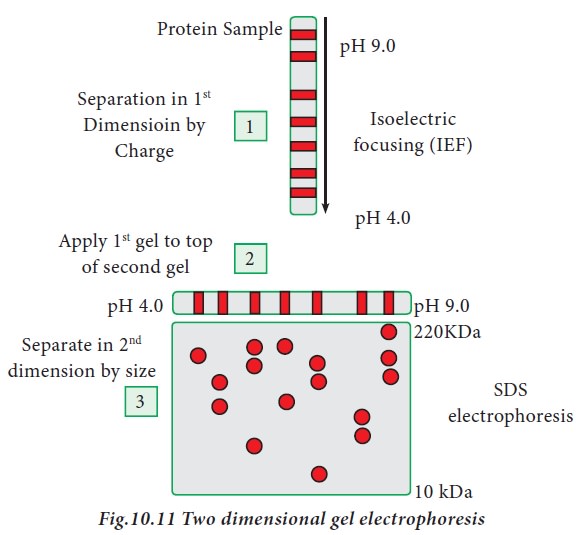
iii. Two -Dimensional gel electrophoresis
Two dimensional gel electrophoresis
was introduced by O’Farell in the year 1975. It is a combination of two
techniques, iso electric focusing and SDS-PAGE. Iso-electric focusing is an
electrophoretic technique where proteins are separated based on their
iso-electric point (pI). pI is the pH at which the amino acid does not migrate
in an electric field (zwitterion form). When a gradient of pH is applied to a
gel, and electric field applied to it, one end becomes more positive than the
other. Relatively at all pH other than its iso electric point, proteins have a
charge (positive or negative) and will be pulled to the opposite side of the
gel. In two dimensional electrophoresis, proteins are separated based on
isoelectric point and molecular mass. To accomplish this, proteins are first
separated by isoelectric focusing where they are separated by their respective
isoelectric point. Second dimension of separation is achieved through SDS-PAGE,
where proteins are separated according to their molecular weight. Each spot on
the resulting 2D gel correspond to single protein species present in the sample
(Fig.10.11)
Applications : SDS–PAGE can be
used to determine the molecular weight of
proteins. To achieve this, a standard mixture of proteins of various
molecular weight (molecular weight ladder) was added for direct comparison of
migration distance. The molecular weights around 15-200kDa can be analyzed in
this manner. Another application of SDS PAGE is to check the purity of a protein
sample. Presence of a single band denote the protein sample is pure.
iv Agarose gel electrophoresis
Agarose is one of the several
components that can be separated from agar. The major source of agar is certain
species of sea weed. Agarose is a linear polymer made up of alternating units
of galactose and 3,6- anhydrogalactose. Agarose gels are completely transparent
when cooled to room temperature.
In Agarose gel electrophoresis, DNA
or RNA molecules can be separated based on their size. This is achieved by the movement of
negatively charged nucleic acid molecules through an agarose matrix in a
horizontal electrophoresis. Molecules with smaller size move faster and migrate
farther as compared to longer ones. The distance between DNA or RNA bands of a
given length is determined by the percentage of agarose in the gel.
Related Topics