Electrostatics - Electric dipole | 12th Physics : Electrostatics
Chapter: 12th Physics : Electrostatics
Electric dipole
Electric dipole
Two equal and opposite
charges separated by a small distance constitute an electric dipole. In many molecules, the
centers of positive and negative charge do not coincide. Such molecules
behave as permanent dipoles. Examples: CO, water, ammonia, HCl etc.
Consider two equal and
opposite point charges (+q, -q) that are separated by a distance 2a as shown in
Figure 1.16(a). The electric dipole moment is defined as
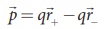
Here ![]() + is the position vector
of +q from the origin and
+ is the position vector
of +q from the origin and ![]() - is the position vector
of -q from the origin. Then, from Figure 1.16 (a),
- is the position vector
of -q from the origin. Then, from Figure 1.16 (a),
The electric dipole
moment vector lies along the line joining two charges and is directed from –q
to +q. The SI unit of dipole moment is coulomb meter (Cm). The electric field
lines for an electric dipole are shown in Figure 1.16 (b).
·
For simplicity, the two charges are placed on the x-axis. Even if
the two charges are placed on y or z-axies, dipole moment will point from –q to
+q. The magnitude of the electric dipole moment is equal to the product of the
magnitude of one of the charges and the distance between them, |![]() |
= 2qa.
|
= 2qa.
·
Though the electric dipole moment for two equal and opposite
charges is defined, it is very general. It is possible to define and calculate
the electric dipole moment for a single charge, two positive charges, two
negative charges and also for more than two charges.
For a collection of n
point charges, the electric dipole moment is defined as follows:

where ![]() i
is the position vector of charge qi from the origin.
i
is the position vector of charge qi from the origin.
EXAMPLE 1.10
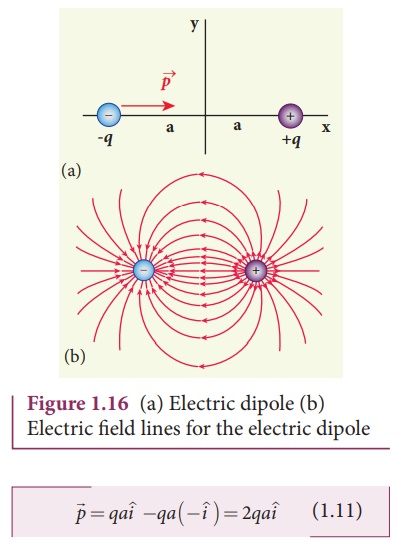
Calculate the electric
dipole moment for the following charge configurations.
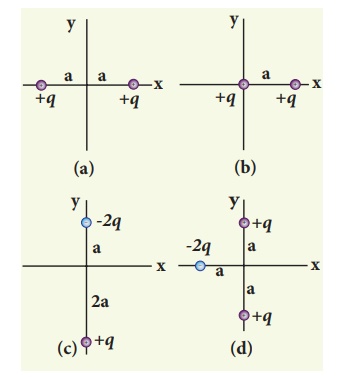
Solution
Case (a) The position vector for the +q on the positive x-axis is ai and
position vector for the +q charge the negative x axis is -a i ^ . So the dipole moment
is,
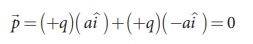
Case (b) In this case one charge is placed at the origin, so its position
vector is zero. Hence only the second charge +q with position vector ai
contributes to the dipole moment, which is ![]() = qa
= qa![]() .
.
From both cases (a) and
(b), we can infer that in general the electric dipole moment depends on the
choice of the origin and charge configuration. But for one special case, the
electric dipole moment is independent of the origin. If the total charge is
zero, then the electric dipole moment will be the same irrespective of the choice
of the origin. It is because of this reason that the electric dipole moment of
an electric dipole (total charge is zero) is always directed from –q to +q,
independent of the choice of the origin.
Case (c) 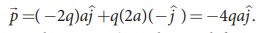 . Note that in
this case p is directed from -2q to +q.
. Note that in
this case p is directed from -2q to +q.
Case (d) 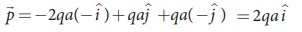
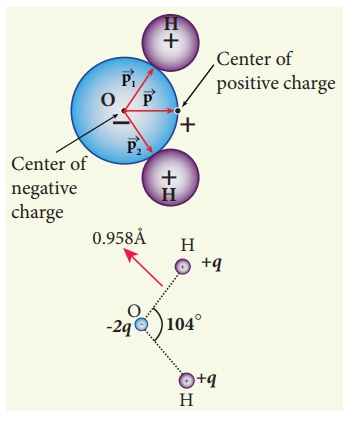
The water molecule (H2O)
has this charge configuration. The water molecule has three atoms (two H atom
and one O atom). The centers of positive (H) and negative (O) charges of a
water molecule lie at different points, hence it possess permanent dipole
moment. The O-H bond length is 0.958 × 10-10 m due to which the
electric dipole moment of water molecule has the magnitude p = 6.1 x 10-30
Cm. The electric dipole moment ![]() is directed from center of
negative charge to the center of positive charge, as shown in the figure.
is directed from center of
negative charge to the center of positive charge, as shown in the figure.
Related Topics