Chapter: Medical Physiology: Physiology of Deep-Sea Diving and Other Hyperbaric Conditions
Effect of High Partial Pressures of Individual Gases on the Body
Effect of High Partial Pressures of Individual Gases on the Body
The individual gases to which a diver is exposed when breathing air are nitro-gen, oxygen, and carbon dioxide; each of these at times can cause significantphysiologic effects at high pressures.
Nitrogen Narcosis at High Nitrogen Pressures
About four fifths of the air is nitrogen. At sea-level pressure, the nitrogen has no significant effect on bodily function, but at high pressures it can cause varying degrees of narcosis. When the diver remains beneath the sea for an hour or more and is breathing compressed air, the depth at which the first symptoms of mild narcosis appear is about 120 feet. At this level the diver begins to exhibit joviality and to lose many of his or her cares.
At 150 to 200 feet, the diver becomes drowsy. At 200 to 250 feet, his or her strength wanes considerably, and the diver often becomes too clumsy to perform the work required. Beyond 250 feet (8.5 atmospheres pressure), the diver usually becomes almost useless as a result of nitrogen narcosis if he or she remains at these depths too long.
Nitrogen narcosis has characteristics similar to those of alcohol intoxication, and for this reason it has frequently been called “raptures of the depths.” The mechanism of the narcotic effect is believed to be the same as that of most other gas anesthetics. That is, it dissolves in the fatty substances in neuronal mem-branes and, because of its physical effect on altering ionic conductance through the membranes, reduces neuronal excitability.
Oxygen Toxicity at High Pressures
Effect of Very High PO2 on Blood Oxygen Transport. When thePO2 in the blood rises above 100 mm Hg, the amount of oxygen dissolved in the water of the blood increases markedly. This is shown in Figure 44–2, which depicts the same oxygen-hemoglobin dissociation curve but with the alveolar PO2 extended to more than 3000 mm Hg. Also depicted by the lowest curve in the figure is the volume of oxygendissolved in the fluid of the blood at each PO2level.Note that in the normal range of alveolar PO2 (below 120 mm Hg), almost none of the total oxygen in the blood is accounted for by dissolved oxygen, but as the oxygen pressure rises into the thousands of millime-ters of mercury, a large portion of the total oxygen is then dissolved in the water of the blood, in addition to that bound with hemoglobin.
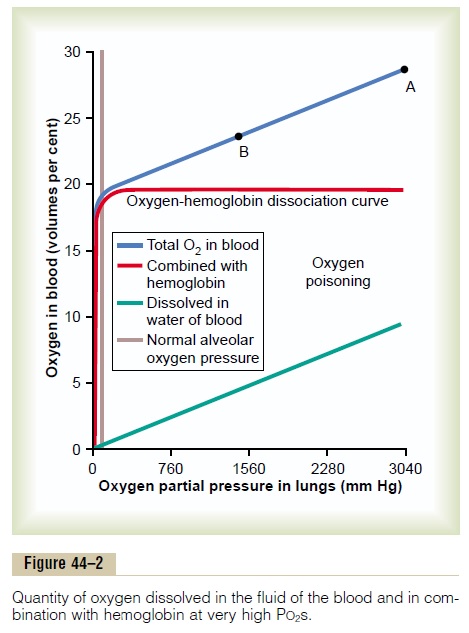
Effect of High Alveolar PO2 on Tissue PO2. Let us assume thatthe PO2 in the lungs is about 3000 mm Hg (4 atmos-pheres pressure). Referring to Figure 44–2, one finds that this represents a total oxygen content in each 100 milliliters of blood of about 29 volumes per cent, as demonstrated by point A in the figure—this means 20 volumes per cent bound with hemoglobin and 9 volumes per cent dissolved in the blood water. As this blood passes through the tissue capillaries and the tissues use their normal amount of oxygen, about 5 milliliters from each 100 milliliters of blood, the oxygen content on leaving the tissue capillaries is still 24 volumes per cent (point B in the figure). At this point, the PO2 is approximately 1200 mm Hg, which means that oxygen is delivered to the tissues at this extremely high pressure instead of at the normal value of 40 mm Hg. Thus, once the alveolar PO2 rises above a critical level, the hemoglobin-oxygen buffer mechanism is no longer capable of keeping the tissue PO2 in the normal, safe range between 20 and 60 mm Hg.
Acute Oxygen Poisoning. The extremely high tissue PO2that occurs when oxygen is breathed at very high alve-olar oxygen pressure can be detrimental to many of the body’s tissues. For instance, breathing oxygen at 4 atmospheres pressure of oxygen (PO2 = 3040 mm Hg) will cause brain seizures followed by coma in most people within 30 to 60 minutes. The seizures often occur without warning and, for obvious reasons, are likely to be lethal to divers submerged beneath the sea.
Other symptoms encountered in acute oxygen poi-soning include nausea, muscle twitchings, dizziness, disturbances of vision, irritability, and disorientation. Exercise greatly increases the diver’s susceptibility to oxygen toxicity, causing symptoms to appear much earlier and with far greater severity than in the resting person.
Excessive Intracellular Oxidation as a Cause of Nervous System Oxygen Toxicity—“Oxidizing Free Radicals.” Molecular oxygen (O2) has little capabilityof oxidizing other chemical compounds. Instead, it must first be converted into an “active” form of oxygen. There are several forms of active oxygen called oxygen free radicals. One of the most important of these is the superoxide free radical O2_, and another is the peroxide radical in the form of hydrogen perox-ide. Even when the tissue PO2 is normal at the level of 40 mm Hg, small amounts of free radicals are contin-ually being formed from the dissolved molecular oxygen. Fortunately, the tissues also contain multiple enzymes that rapidly remove these free radicals, including peroxidases, catalases, and superoxide dis-mutases. Therefore, so long as the hemoglobin-oxygenbuffering mechanism maintains a normal tissue PO2, the oxidizing free radicals are removed rapidly enough that they have little or no effect in the tissues.
Above a critical alveolar PO2 (above about 2 atmospheres PO2), the hemoglobin-oxygen buffering mechanism fails, and the tissue PO2 can then rise to hundreds or thousands of millimeters of mercury. At these high levels, the amounts of oxidizing free radi-cals literally swamp the enzyme systems designed to remove them, and now they can have serious destruc-tive and even lethal effects on the cells. One of the principal effects is to oxidize the polyunsaturated fatty acids that are essential components of many of the cell membranes. Another effect is to oxidize some of the cellular enzymes, thus damaging severely the cellular metabolic systems. The nervous tissues are especially susceptible because of their high lipid content.
Therefore, most of the acute lethal effects of acute oxygen toxicity are caused by brain dysfunction.
Chronic Oxygen Poisoning Causes Pulmonary Disabil- ity. A person can be exposed to only 1 atmospherepressure of oxygen almost indefinitely without devel-oping the acute oxygen toxicity of the nervous system just described. However, after only about 12 hours of 1 atmosphere oxygen exposure, lung passageway con-gestion, pulmonary edema, and atelectasis caused bydamage to the linings of the bronchi and alveoli begin to develop. The reason for this effect in the lungs but not in other tissues is that the air spaces of the lungs are directly exposed to the high oxygen pressure, but oxygen is delivered to the other body tissues at almost normal PO2because of the hemoglobin-oxygen buffer system.
Carbon Dioxide Toxicity at Great Depths in the Sea
If the diving gear is properly designed and functions properly, the diver has no problem due to carbon dioxide toxicity because depth alone does not increase the carbon dioxide partial pressure in the alveoli. This is true because depth does not increase the rate of carbon dioxide production in the body, and as long as the diver continues to breathe a normal tidal volume and expires the carbon dioxide as it is formed, alveo-lar carbon dioxide pressure will be maintained at a normal value.
In certain types of diving gear, however, such as the diving helmet and some types of rebreathing appara-tuses, carbon dioxide can build up in the dead space air of the apparatus and be rebreathed by the diver. Up to an alveolar carbon dioxide pressure (PCO2) of about 80 mm Hg, twice that in normal alveoli, the diver usually tolerates this buildup by increasing the minute respiratory volume a maximum of 8- to 11-fold to compensate for the increased carbon dioxide. Beyond 80-mm Hg alveolar PCO2, the situation becomes intolerable, and eventually the respiratory center begins to be depressed, rather than excited, because of the negative tissue metabolic effects of high PCO2. The diver’s respiration then begins to fail rather than to compensate. In addition, the diver develops severe respiratory acidosis, and varying degrees of lethargy, narcosis, and finally even anesthesia.
Decompression of the Diver After Excess Exposure to High Pressure
When a person breathes air under high pressure for a long time, the amount of nitrogen dissolved in the body fluids increases. The reason for this is the fol-lowing: Blood flowing through the pulmonary capil-laries becomes saturated with nitrogen to the same high pressure as that in the alveolar breathing mixture.
And over several more hours, enough nitrogen is carried to all the tissues of the body to raise their tissue PN2 also to equal the PN2 in the breathing air.
Because nitrogen is not metabolized by the body, it remains dissolved in all the body tissues until the nitro-gen pressure in the lungs is decreased back to some lower level, at which time the nitrogen can be removed by the reverse respiratory process; however, this removal often takes hours to occur and is the source of multiple problems collectively called decompressionsickness.
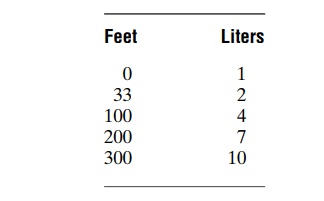
Volume of Nitrogen Dissolved in the Body Fluids at Different Depths. At sea level, almost exactly 1 liter of nitrogenis dissolved in the entire body. Slightly less than one half of this is dissolved in the water of the body and a little more than one half in the fat of the body. This is true because nitrogen is five times as soluble in fat as in water.
After the diver has become saturated with nitrogen, the sea-level volume of nitrogen dissolved in the body at different depths is as follows:
Several hours are required for the gas pressures of nitrogen in all the body tissues to come nearly to equi-librium with the gas pressure of nitrogen in the alveoli. The reason for this is that the blood does not flow rapidly enough and the nitrogen does not diffuse rapidly enough to cause instantaneous equilibrium. The nitrogen dissolved in the water of the body comes to almost complete equilibrium in less than 1 hour, but the fat tissue, requiring five times as much transport of nitrogen and having a relatively poor blood supply, reaches equilibrium only after several hours. For this reason, if a person remains at deep levels for only a few minutes, not much nitrogen dissolves in the body fluids and tissues, whereas if the person remains at a deep level for several hours, both the body water and body fat become saturated with nitrogen.
Decompression Sickness (Synonyms: Bends, Compressed Air Sickness, Caisson Disease, Diver’s Paralysis, Dysbarism). If adiver has been beneath the sea long enough that large amounts of nitrogen have dissolved in his or her body and the diver then suddenly comes back to the surface of the sea, significant quantities of nitrogen bubbles can develop in the body fluids either intracellularly or extracellularly and can cause minor or serious damage in almost any area of the body, depending on the number and sizes of bubbles formed; this is called decompression sickness.
The principles underlying bubble formation are shown in Figure 44–3. In Figure 44–3A, the diver’s tissues have become equilibrated to a high dissolved
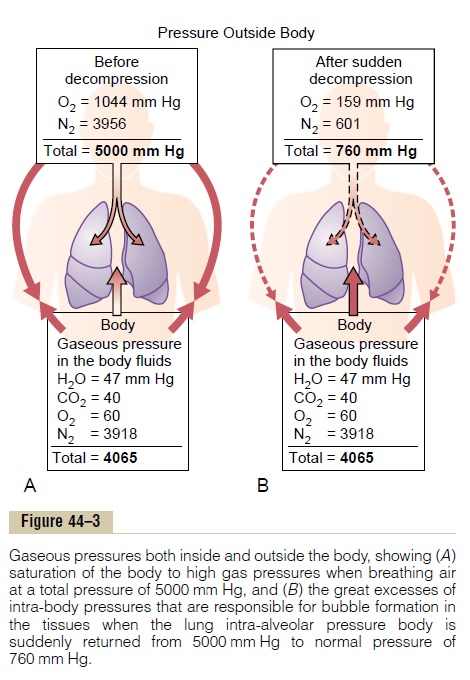
nitrogen pressure (PN2=3918 mm Hg), about 6.5times the normal amount of nitrogen in the tissues. As long as the diver remains deep beneath the sea, the pressure against the outside of his or her body (5000 mm Hg) compresses all the body tissues suffi-ciently to keep the excess nitrogen gas dissolved. But when the diver suddenly rises to sea level (Figure 44–3B), the pressure on the outside of the body becomes only 1 atmosphere (760 mm Hg), while the gas pressure inside the body fluids is the sum of the pressures of water vapor, carbon dioxide, oxygen, and nitrogen, or a total of 4065 mm Hg, 97 per cent of which is caused by the nitrogen. Obviously, this total value of 4065 mm Hg is far greater than the 760 mm Hg pressure on the outside of the body. Therefore, the gases can escape from the dissolved state and form actual bubbles, composed almost entirely of nitrogen, both in the tissues and in the blood where they plug many small blood vessels. The bubbles may not appear for many minutes to hours, because sometimes the gases can remain dissolved in the “supersaturated” state for hours before bubbling.
Symptoms of Decompression Sickness (“Bends”). The symptoms of decompression sickness are caused by gas bubbles blocking many blood vessels in different tissues. At first, only the smallest vessels are blocked by minute bubbles, but as the bubbles coalesce, progressively larger vessels are affected.
Tissue ischemia and sometimes tissue death are the result.
In most people with decompression sickness, the symptoms are pain in the joints and muscles of the legs and arms, affecting 85 to 90 per cent of those persons who develop decompression sickness. The joint pain accounts for the term “bends” that is often applied to this condition.
In 5 to 10 per cent of people with decompression sickness, nervous system symptoms occur, ranging from dizziness in about 5 per cent to paralysis or col-lapse and unconsciousness in as many as 3 per cent. The paralysis may be temporary, but in some instances, damage is permanent.
Finally, about 2 per cent of people with decompres-sion sickness develop “the chokes,” caused by massive numbers of microbubbles plugging the capillaries of the lungs; this is characterized by serious shortness of breath, often followed by severe pulmonary edema and, occasionally, death.
Nitrogen Elimination from the Body; Decompression Tables. Ifa diver is brought to the surface slowly, enough of the dissolved nitrogen can usually be eliminated by expi-ration through the lungs to prevent decompression sickness. About two thirds of the total nitrogen is lib-erated in 1 hour and about 90 per cent in 6 hours.
Decompression tables have been prepared by the U.S. Navy that detail procedures for safe decompres-sion. To give the student an idea of the decompression process, a diver who has been breathing air and has been on the sea bottom for 60 minutes at a depth of 190 feet is decompressed according to the following schedule:
10 minutes at 50 feet depth
17 minutes at 40 feet depth
19 minutes at 30 feet depth
50 minutes at 20 feet depth
84 minutes at 10 feet depth
Thus, for a work period on the bottom of only 1 hour, the total time for decompression is about 3 hours.
Tank Decompression and Treatment of Decompression Sick-ness. Another procedure widely used for decompres-sion of professional divers is to put the diver into a pressurized tank and then to lower the pressure grad-ually back to normal atmospheric pressure, using essentially the same time schedule as noted above.
Tank decompression is even more important for treating people in whom symptoms of decompression sickness develop minutes or even hours after they have returned to the surface. In this case, the diver is recompressed immediately to a deep level. Then decompression is carried out over a period several times as long as the usual decompression period.
“Saturation Diving” and Use of Helium-Oxygen Mixtures in Deep Dives. When divers must work at very deep levels—between 250 feet and nearly 1000 feet—they fre-quently live in a large compression tank for days or weeks at a time, remaining compressed at a pressure level near that at which they will be working. This keeps the tissues and fluids of the body saturated with the gases to which they will be exposed while diving. Then, when they return to the same tank after working, there are no significant changes in pressure, so that decompression bubbles do not occur.
In very deep dives, especially during saturation diving, helium is usually used in the gas mixture instead of nitrogen for three reasons: (1) it has only about one fifth the narcotic effect of nitrogen; (2) only about one half as much volume of helium dissolves in the body tissues as nitrogen, and the volume that does dissolve diffuses out of the tissues during decompres-sion several times as rapidly as does nitrogen, thus reducing the problem of decompression sickness; and (3) the low density of helium (one seventh the density of nitrogen) keeps the airway resistance for breathing at a minimum, which is very important because highly compressed nitrogen is so dense that airway resistance can become extreme, sometimes making the work of breathing beyond endurance.
Finally, in very deep dives it is important to reduce the oxygen concentration in the gaseous mixture because otherwise oxygen toxicity would result. For instance, at a depth of 700 feet (22 atmospheres of pressure), a 1 per cent oxygen mixture will provide all the oxygen required by the diver, whereas a 21 per cent mixture of oxygen (the percentage in air) deliv-ers a PO2 to the lungs of more than 4 atmospheres, a level very likely to cause seizures in as little as 30 minutes.
Related Topics