Chapter: Biology of Disease: Disorders of Water, Electrolytes and Urate Balances
Disorders of K+ Homeostasis
DISORDERS OF K+ HOMEOSTASIS
Potassium ions are necessary to maintain cell volume, for the
optimal activities of a number of enzymes, and to maintain the resting
potential of cell membranes and therefore neuromuscular functions, especially
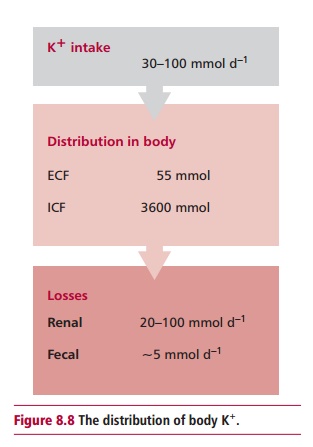
in the heart . The intake of K+ varies between 30 and 100 mmol day –1
and losses are equally variable. The kidneys excrete most ingested K+
with a smaller amount being eliminated by the GIT. A high concentration of
plasma K+ stimulates the release of aldosterone that, in turn,
increases the renal excretion of K+. Gastrointestinal losses can be
significant during vomiting and diarrhea. Only very small amounts of K+
are lost in sweat. The average 70 kg human contains about 3600 mmol of K+(Figure 8.8), almost all being found in the ICF. Values for the
concentration of K+ in the serum below and above the reference range
of 3.4 to 4.9 mmol dm–3 are called hypokalemia and hyperkalemia
respectively. Hyperkalemia is the more common clinical condition.
Unlike Na+, the plasma K+ concentration
does not vary significantly with water loss or overload. However, hyperkalemia
must be identified because concentrations of serum K+above 7 mmol dm–3
can result in cardiac arrest and death. Renal failure, acidosis, aldosterone
deficiency, damage to cells and an excess intake of K+ can all cause
hyperkalemia. In renal failure, the kidneys are unable to excrete K+
because of the low GFR. Further, acidosis, a common feature of renal failure,
leads to hyperkalemia because the low pH of the ECF means that K+
moves out of cells in exchange for H+, to return the pH to reference
values. A deficiency of aldosterone, such as in Addison’s disease where the
kidneys lose their ability to excrete K+, can result in
hyperkalemia. The destruction of cells during trauma can release large amounts
of K+ causing hyperkalemia. Lastly, an excessive oral or parenteral
intake of K+ is a rare cause of hyperkalemia. The treatment of
hyperkalemia includes infusion of insulin and glucose to promote the entry of K+
into cells. Severe hyperkalemia may require dialysis.
Hypokalemia is clinically significant, giving rise to muscular
weakness and cardiac arrhythmias, hence patients often present with
breathlessness and chest pain. The causes of hypokalemia include increased K+
losses from the GIT or kidneys, alkalosis, certain clinical disorders, some
drugs, or a decreased K+ intake. Excessive losses from the GIT can
occur during vomiting and diarrhea. Hypokalemia occurs in alkalosis because the
pH of the ECF is high and H+ moves from the ICF to the ECF as part
of the buffering process, while K+ moves in the opposite direction
leading to hypokalemia. A number of disorders, for example, Cushing’s and
Conn’s syndromes, are associated with increased cortisol and aldosterone
production respectively. Both hormones have mineralocorticoid activity and
stimulate the renal retention of Na+ in exchange for K+
causing hypokalemia. Drugs, such as carbenoxolone used to treat gastric ulcers
, can cause hypokalemia because of their mineralocorticoid activity. Decreases
in oral or parenteral intakes of K+ are rare but can lead to
hypokalemia. Patients with hypokalemia are treated with oral K+
salts. Severe hypokalemia may require intravenous infusions of K+.
Related Topics