Chapter: Medical Physiology: Digestion and Absorption in the Gastrointestinal Tract
Digestion of Proteins
Digestion of Proteins
Proteins of the Diet. The dietary proteins are chemicallylong chains of amino acids bound together by peptidelinkages. A typical linkage is the following:
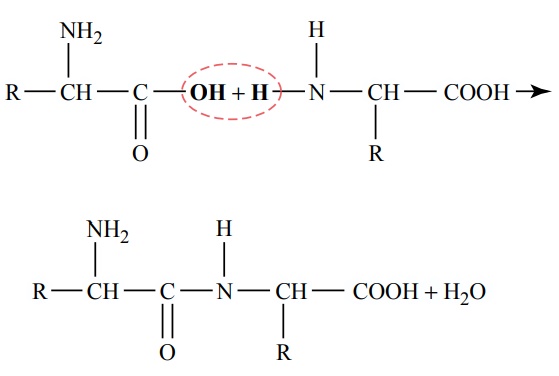
The characteristics of each protein are determined by the types of amino acids in the protein molecule and by the sequential arrangements of these amino acids. The physical and chemical characteristics of different proteins important in human tissues are discussed.
Digestion of Proteins in the Stomach. Pepsin, the importantpeptic enzyme of the stomach, is most active at a pH of 2.0 to 3.0 and is inactive at a pH above about 5.0. Consequently, for this enzyme to cause digestive action on protein, the stomach juices must be acidic. The gastric glands secrete a large quantity of hydrochloric acid. This hydrochlo-ric acid is secreted by the parietal (oxyntic) cells in the glands at a pH of about 0.8, but by the time it is mixed with the stomach contents and with secretions from the nonoxyntic glandular cells of the stomach, the pH then averages around 2.0 to 3.0, a highly favorable range of acidity for pepsin activity.
One of the important features of pepsin digestion is its ability to digest the protein collagen, an albuminoid type of protein that is affected little by other digestive enzymes. Collagen is a major constituent of the inter-cellular connective tissue of meats; therefore, for the digestive enzymes of the digestive tract to penetrate meats and digest the other meat proteins, it is first nec-essary that the collagen fibers be digested. Conse-quently, in persons who lack pepsin in the stomach juices, the ingested meats are less well penetrated by the other digestive enzymes and, therefore, may be poorly digested.
As shown in Figure 65–2, pepsin only initiates the process of protein digestion, usually providing only 10 to 20 per cent of the total protein digestion to convert the protein to proteoses, peptones, and a few polypep-tides. This splitting of proteins occurs as a result of hydrolysis at the peptide linkages between amino acids.
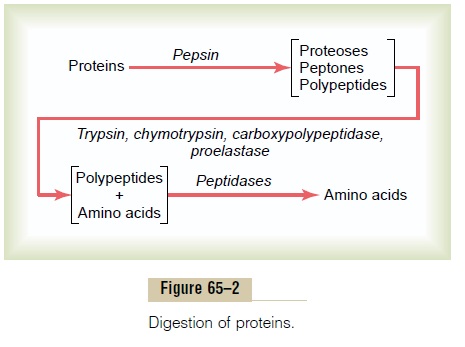
Digestion of Proteins by Pancreatic Secretions. Mostprotein digestion occurs in the upper small intestine, in the duodenum and jejunum, under the influence of proteolytic enzymes from pancreatic secretion. Immediately on entering the small intestine from the stomach, the partial breakdown products of the protein foods are attacked by major proteolytic pancreatic enzymes: trypsin, chymotrypsin,carboxy-polypeptidase, and proelastase, as shown in Figure 65–2.
Both trypsin and chymotrypsin split protein mole-cules into small polypeptides; carboxypolypeptidase then cleaves individual amino acids from the carboxyl ends of the polypeptides. Proelastase, in turn, is con-verted into elastase, which then digests elastin fibers that partially hold meats together.
Only a small percentage of the proteins are digested all the way to their constituent amino acids by the pancreatic juices. Most remain as dipeptides and tripeptides.
Digestion of Peptides by Peptidases in the Enterocytes That Line the Small Intestinal Villi. The last digestive stage ofthe proteins in the intestinal lumen is achieved by the enterocytes that line the villi of the small intestine, mainly in the duodenum and jejunum. These cells have abrush border that consists of hundreds of microvilli projecting from the surface of each cell. In the mem-brane of each of these microvilli are multiple pepti-dases that protrude through the membranes to theexterior, where they come in contact with the intestinal fluids.
Two types of peptidase enzymes are especially important, aminopolypeptidase and several dipepti-dases. They succeed in splitting the remaining largerpolypeptides into tripeptides and dipeptides and a few into amino acids. Both the amino acids plus the dipep-tides and tripeptides are easily transported through the microvillar membrane to the interior of the enterocyte.
Finally, inside the cytosol of the enterocyte are multiple other peptidases that are specific for the remaining types of linkages between amino acids. Within minutes, virtually all the last dipeptides and tripeptides are digested to the final stage to form single amino acids; these then pass on through to the other side of the enterocyte and thence into the blood.
More than 99 per cent of the final protein digestive products that are absorbed are individual amino acids, with only rare absorption of peptides and very, very rare absorption of whole protein molecules. Even these very few absorbed molecules of whole protein can sometimes cause serious allergic or immunologic disturbances.
Related Topics