Chapter: Aquaculture Principles and Practices: Design and Construction of Aquafarms
Design and construction of hatcheries
Design and construction of hatcheries
Methods of seed production in aquaculture vary considerably with the species under culture and the state of technology, as well as with the level of operation (as, for example, extensive or intensive and the number or frequency of crops). Where the techniques for arti ficial propagation are still to be developed or perfected, or where it is feasible and economical to collect eggs, larvae or fry from natural breeding areas, sophisticated hatchery systems are not often used. However, even in such cases it is generally accepted that, eventually, hatchery production of seed will be necessary to stabilize and ensure regular supplies and introduce breeding techniques to raise improved seed for better growth and production.
As is only to be expected, there are different types of hatchery facilities in use, depending on the species, locality and investment capabilities of the aquaculturists. However, the basic requirements are about the same: there has to bethe necessary facilities for holding or rearing an adequate brood stock, spawning or stripping and fertilization of ova, incubation of fertilized ova and rearing of larvae to the required stage for transfer to nurseries or other culture facilities.
Source and supply of water
The selection of a suitable site for a hatchery is very important for its successful operation. Although, for various reasons, it is preferable to have it located near the grow-out farm, often a different site may have to be selected because of the water quality and quantity requirements. There are also cases where the hatchery forms an independent enterprise or is meant to produce seed for a number of grow-out farms. In principle, surface or ground water can be used in hatcheries, if it satisfies the necessary water quality criteria. Surface water from streams, rivers, lakes and the open sea may be relatively less expensive to utilize. However, very often there will be the need to filter the water, and where there is a high content of silt it may be necessary to have a settling tank. Generally, sand or gravel filters with backflushing will make the water suitable for the hatchery. In salmon and trout hatcheries, where stricter water quality conditions are maintained, spring or borewell water is preferred, to eliminate the risk of contamination. As far as possible the source of water, or at least the entire course of the water supply system from the intake, should be under the control of the hatchery manager. Well water often has an excess of gas, which can cause gas bubble disease, but through adequate aeration (fig. 6.35) before use in the hatchery, this problem can be overcome. A large reservoir of properly aerated water from a spring can be a suitable source for a controlled water supply to a hatchery.
Water temperature is of special importance in a hatchery system, as the maturation of the brood stock, spawning, development of fertilized ova and growth of larvae are all directly affected by it. Spring water has often the advantage of constant temperature conditions. The temperature to be maintained in different units of the hatchery installation will depend on the requirements of the propagated species. It may be necessary or desirable to have provisions for

regulating the temperature, as for example by mixing cold and warm water from separate supply lines or by the provision of an inline heat exchanger, including a thermostatically controlled boiler which can be by-passed if heat control is not needed. While 20–30°C is generally the temperature requirement of warm water fishes, trout and salmon hatcheries maintain a temperature between 7 and 15°C. In tropical shrimp hatcheries, a temperature ranging between 20 and 29°C is suitable, but for the majority of species a temperature not lower than 25°C is considered optimal. In the giant fresh-water prawn hatchery a higher temperature of 30–31°C is recommended for better growth and survival. In oyster hatcheries a temperature of about 29°C is maintained.
Dissolved oxygen and pH are other impor-tant properties of the water for hatcheries. The lowest safe level of dissolved oxygen for trout hatcheries is about 5 ppm, but a higher concentration of 7 ppm is preferred. According to Wickins (1981), salmonids and warm-water crustacea should not be exposed to levels of dis-solved oxygen below 5 mg/l for more than a few hours. Equivalent levels for eels and carp range from 3 to 4 mg/l. In other warm water species of fish and shrimp, slightly lower oxygen contents may be adequate. Oxygenation of water in a hatchery is relatively simple and is generally achieved by the manipulation of water flow from the source or the use of appropriate aer-ating devices. In salt-water hatcheries, maintenance of the required salinity can be important, although many species are quite tolerant of fluctuations within limits. In order to be able to regulate salinity when required, salt-water hatcheries generally maintain supplies of fresh water as well as sea water.
Reconditioning and recirculation of water
Where the availability of good-quality water is limited, hatcheries have to resort to recondi-tioning and recirculation. In certain circumstances, it may also be considered necessary to reduce risks of infection by pathogens and parasites through continued use of water from external sources. When the water has to pass through a series of tanks, it has often been the practice in hatcheries to pump the water through an aerator, after it has passed through a certain number of tanks, before further distribution. Naturally, there are intrinsic dangers in such simple systems of recirculation. Though oxygen can be replenished through aeration and most of the carbon dioxide dissipated, the removal of metabolic products like ammonia will involve more complex systems, which besides reaeration and mechanical filtration may involve biological treatment. Recent designs of semi-closed systems employ one or more by-pass treatment units, such as for deni-trification, oxygenation, ozonization, etc. In principle, such recirculation should make it eco-nomically feasible to grow warm-water species in temperate climates by reducing the cost of heating. In practice, there are many constraints to its application in commercial aquaculture, but it can be used in hatchery situations, when essential.
Ammonia can be removed by nitrifying bacteria. Ammonia is first converted primarily by Nitrosomonas to nitrous acid, then by Nitrobacter to nitric acid. The acid combines with anavailable base to form nitrites and then nitrates. Nitrates are harmless in the recirculating system. Even in prolonged exposures in culture systems, no toxic effects have been reported below 100 mg/l nitrate nitrogen (Wickins, 1981). Nitrite toxicity is influenced by water chemistry, but it has been suggested that concentrations in hard fresh water should not exceed 0.1 mg/l nitrite nitrogen, and in sea water 1.0 mg/l nitrite nitrogen.
There are several systems and designs employed in waste-water treatment for hatchery use as well as for intensive aquaculture (seeRecirculating of aquaculture tank production systems Tank farms). Many ofthem have been described in literature (Tiews, 1981; Bovendeur et al., 1987; Heinsbroek and Kamstra, 1990; Losardo et al., 2001; Bergheim and Brinker, 2003). The most commonly used and relatively more economic treatment would appear to be biofiltration, which may incorporate downflow filters (e.g. trickling filters), upflow filters or horizontal flow filters. Several types of filter media are in use, such as sand, gravel, oyster shells, plastics, anthracite, activated carbon, diatomaceous earth and their combinations. Besides serving as strainers, they provide surface area for biological growth. Through biological growth and oxidation, ammonia is converted into nitrite and nitrate. The nitrate may be further combined with ions in water to form salts or reduced to nitrogen gas through a denitrification process. According to Liao and Mayo (1974), with a retention time of about 30 minutes and a hydraulic loading rate of 1.0–3.7 l/s per m2, about 48 per cent of the initial ammonia load can be removed.
A rotating biofilter process is employed as a secondary treatment. The basic unit consists of a half-cylinder tank, through the ends of which a horizontal shaft is mounted. As waste water flows through the cylinder, the discs, which are half submerged, are rotated. A layer of micro-organisms grows on the discs and acts as the aerobic biochemical agent to remove the dissolved wastes from the water. By arranging the discs in a series of stages, the rate of oxidation of the organic materials is increased by improved residence time. A surface area loading of 0.06–0.1 m3/day per m2 will be needed to achieve a better than 95 per cent removal efficiency for biological oxygen demand (BOD) decrease and nitrate nitrogen.
Ion exchange is an efficient and reliable means of ammonia removal, but it is much more expensive than biological filtration. Clinoptilolite, one of the zeolites used in water treatment, is an effective natural ion exchange material for the removal of ammonia from hatchery water. It has a high selectivity for ammonia. The minimum depth of an ion exchange bed is 0.61–0.76 m with a flow rate in the range 1.4–3.4 l/sec per m2 (Liao, 1981). The regeneration is achieved by passing 5–10 per cent brine solution through the exchange bed, with a flow of 0.68–1.36 l/sec per m2.
Although in principle it is possible to use aquatic plants, including algae, to remove metabolites and nutrients from water through assimilation or biological conversion, there are practical difficulties, particularly for use in connection with a hatchery system. Another means of reconditioning water is through the use of an activated sludge process, in which biological oxidation occurs in the fluidized suspension of the sludge. Mention may also be made of the use of ozone, which is a very strong oxidizing agent and oxidizes the organics in the water and at the same time serves as a disinfectant. However, its use in hatcheries has not progressed beyond the experimental stage.
In all these systems there is the need for a regular supply of make-up water, at a rate of at least 5–7 per cent of the total volume, to replace the losses due to filter back-flushing, draining of the sludge and evaporation.
Hatchery equipment
Besides the installations for an adequate supply of water of the required quality, the other general equipment required in a hatchery includes tanks for brood stock; implements for collecting and handling breeders, for stripping and fertilization; spawning tanks where neces sary, jars, troughs, tanks or other containers, net cages or ‘hapas’ (mesh cloth tanks) for incubating and hatching fertilized eggs; food dispensers; larval rearing tanks and aeration systems.
The brood tanks are very similar to the ones described. There are a variety of implements used for collection, handling and transport of breeders in the hatchery. The main consideration is to avoid damage during collection and transport. In the case of large finfish, special hammocks have proved very efficient (fig. 6.36). Scoop nets are commonly used for catching brood stock for spawning.

In shrimp hatcheries, circular maturation tanks of about 12 m3 capacity made of fibreglass or cement concrete are used. The substrate consists of a layer of gravel (about 10 cm thick) separated from a 5–10 cm thick second layer of coral sand by a permeable synthetic cloth to prevent gravel from mixing with sand (AQUACOP, 1983). Concentric perforated plastic pipes fitted in a PVC tube (10 cm diameter) are embedded in the gravel. Water that flows into these pipes passes through the sand, preventing the accretion of wastes and sediments in the sand. Water is discharged through two concentric tubes, allowing the bottom water to be drained first. Water exchange is achieved two or three times a day.

Spawning tanks in the open may be built of concrete, with adequate arrangements for water circulation, as in the case of Chinese carp hatcheries (fig. 6.37). In shrimp hatcheries, indoor tanks of concrete or fibreglass are commonly used for spawning (fig. 6.38). Cylindro-conical, 150 l capacity fibreglass tanks have been found to be very convenient (AQUACOP,1983). The overflow passes through a concentrator provided with a 100 mm mesh that retains the eggs. A perforated plastic plate fitted over the conical bottom prevents the spawners from eating the accumulated eggs.

Different types and designs of incubators are used for hatching fertilized eggs, ranging from improvised earthen and polyethylene jars to sophisticated batteries of jars and troughs. To a certain extent, the degree of sophistication depends on the species, the size of the eggs and the magnitude of operations. However, thegeneral principle involved is the provision of a regulated flow of good quality water of the required temperature, for the development and hatching of fertilized eggs and prevention of infections that will affect the hatching rate.

Troughs made of wood, concrete, aluminium, plastic or fibreglass are commonly used in many types of hatcheries (figs 6.39 and 6.40). The size varies, but an average size may be about 3 m x 0.5 m x 0.25 m. They are generally screened at the intake to prevent the entry of detritus and at the outflow to prevent the escape of larvae.

By the use of vertically adjustable screens, the depth of water in the trough can be regulated. As an alternative, the water depth can be regulated by adjustable elbow pipes.
In the case of trough-type incubators, such as the ones used in trout and salmon hatcheries, there are egg baskets fitted in the tray. The perforations are of the shape and size to retain the eggs, but allow the hatchlings to fall through to the water below in the trough (fig. 6.41). In order to allow aeration of the eggs, water is forced upward through the perforations.


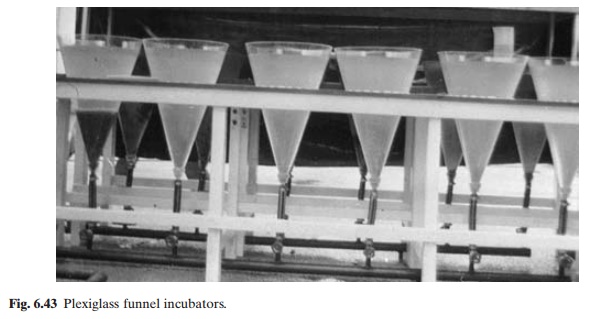
Special glass jar incubators (known as Zuger jars, Weiss jars or Zug-Weiss jars and MacDonald jars) and plexiglass or other plastic funnels (figs 6.42 and 6.43) as well as less expen-sive sieve-cloth funnels and even earthen jars are used for incubating non-adhesive eggs (figs 6.44 and 6.45). Even in cases like the common carp, these devices can be used after removing the sticky layer.


In oyster hatcheries, mature male and female oysters are spawned individually in battery jars containing filtered water of the required temperature (about 27°C). The spawned eggs and milt are filtered out and the ova fertilized. Larval tanks are provided for the development and hatching of the ova and for rearing through various larval stages.
Different types of larval rearing troughs, tanks and pools are in use and some types are readily available from manufacturers. The basic requirements are proper circulation and drainage of water to keep them well supplied

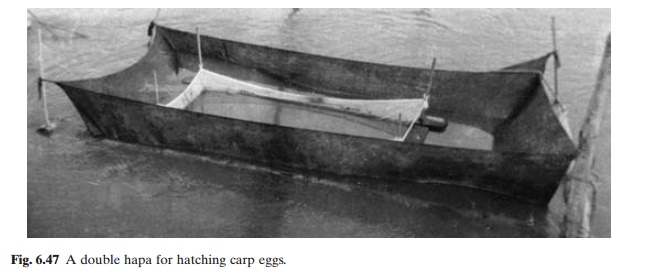
with clean oxygenated water and prevent accumulation of waste products. For rearing carp larvae, circular cement pools built on the ground are commonly used. For hatching and larval rearing in Indian carp culture, meshed cloth tanks (hapas) fixed on the pond bottom by means of stakes (fig. 6.46) are widely used. It is common to have double tanks (fig. 6.47). The inner smaller tank with fine mesh holds the fertilized eggs for hatching. The hatched larvae fall into the outer larger cage, leaving behind the egg shells and debris. The inner cage can be removed easily after hatching. Rectangular cement cisterns, with an adequate water supply and drainage, are also used in many places.
Layout and accessories
Although some of the simple and improvised hatchery systems such as the carp hatcheries in China and India are built in the open, modern hatcheries are installed indoors. In some cases the larval rearing may be carried out in tanks, pools or ponds outdoors, but where water temperature has to be ontrolled they are generally provided with at least a protective roofing.
There are many ways of arranging the installations in a hatchery. Some have maturation, spawning and fertilization, hatching and larval rearing in different sections. Others have most of them in one area, particularly when there are limitations of space. An important factor that would determine the arrangement of the equipment is the convenience of water distribution and drainage. The main supply channel or pipe could run along one wall of the hatchery building or along its centre. The latter is more common, when the tanks and incubators can be installed on either side of the supply system, with drains along the walls. The size of the water supply system should be adequate to carry one and a half times the quantity of water required for operation of the hatchery. When siting supply lines, it will be advisable to take into account possible future expansion of the hatchery. An overhead reservoir or a head tank from which the water flows by gravity will be very useful in maintaining a constant water pressure in the system and consequently a uniform flow in the hatching units. It also serves as a standby in times of power failure.
In order to save space and reduce the use of water, it is possible to stack the troughs or trays used for incubation and larval rearing one above the other. Battery incubators are available from manufacturers. They consist of vertically stacked troughs, each having an egg tray and a cover. Each trough can be pulled out separately for inspection. The water flow will pass downwards through each vertical trough stack, trickling through each one from top to bottom.
As indicated earlier, control of water temperature is an important factor in all stages of hatchery operation. If the natural supplies of water need to be heated or cooled for maintaining the required temperature, it will be necessary to install equipment for this purpose. Electrical heating and cooling would probably be the best, but economic considerations and availability may make it necessary to look to other sources such as heated water from industries and power stations. Most hatcheries would require some type of aeration system. Air blowers and air stones are commonly used to provide the aeration that will be needed to meet the extra oxygen requirements in brood stock and nursery tanks.
Suitable means of dispensing feed in maturing and larval tanks have to be provided. Different types of automatic feeders are available, but in many hatcheries manual feeding is still the common practice. In crustacean and mollusc hatcheries, there is also the need to have facilities for algal culture to feed the larvae, besides an artificially lit room formaintaining algal cultures. Large concrete or fibreglass tanks can be used to grow the algal food required. The algal tanks can be outdoors, but if kept indoors there has to be adequate lighting. The tanks should also be provided with proper aeration through air stones or other devices. Shrimp hatcheries may also require similar tanks for hatching Artemia cysts, for feeding the larvae.
Besides suitable storage space for feeds, it is desirable to have, in large hatcheries, some laboratory space for routine tests and examinations.
Related Topics