Chapter: 11th Biochemistry : Chapter 3 : Proteins
Denaturation and Protein folding
Denaturation and Protein folding
Each protein has a unique three
dimensional structure. Upon changes in various factors like temperature, pH,
ionic strength or exposure to certain chemicals like urea lead to disruption of
its three-dimensional structure and turn back into an unstructured string of
amino acids. When a protein loses its higher-order structure, but not its
primary structure, it is said to be denatured. Denatured proteins are not
functional.
For some proteins, denaturation can
be reversed. Since the primary structure of the polypeptide is still intact it
may be able to re-fold into its original structure, if it is returned to its
normal environment. Many proteins do not fold by themselves, but instead get
assistance from other proteins like chaperons.
The process
by which a polypeptide chain acquires its 3-dimensional structure is known as
protein folding. It is a complex process and the exact mechanism by which a
protein folds to its three dimensional structure has not been understood so
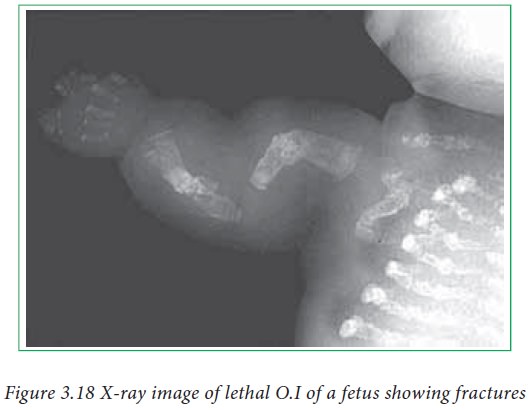
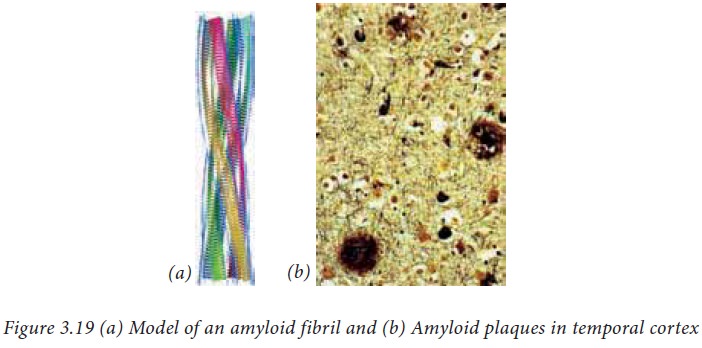
far. Quite often many proteins misfold. Some proteins when misfolded form
fibrillar structure of pleated sheets. This misfolding can be spontaneous or
could be because of mutations. These misfolded proteins aggregate in neurons
and can lead to amyloid disease such as Alzheimer’s disease, which is a neuro degenerative disease.
Related Topics